Africa's Emerging Biopharma Frontier


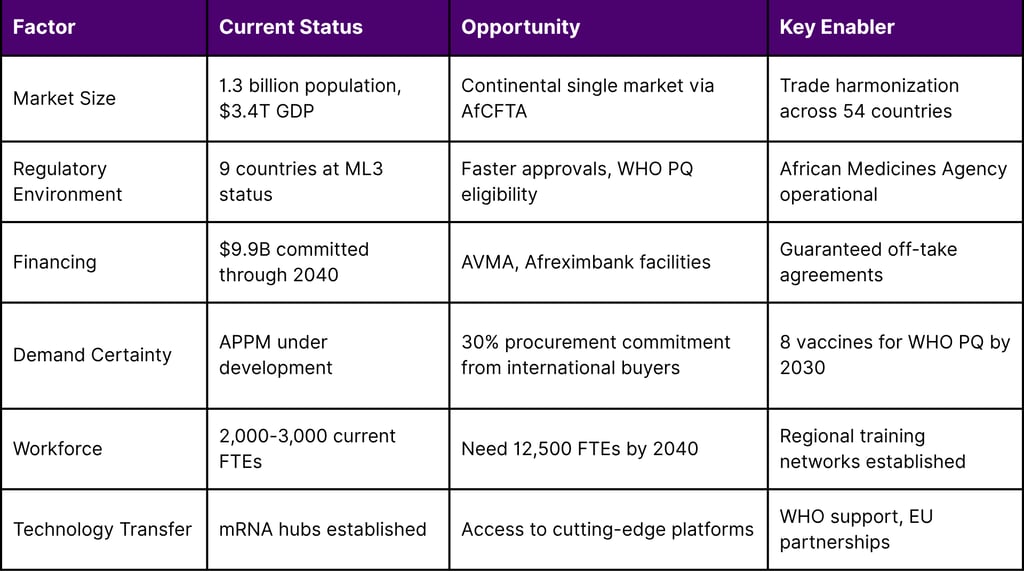
Africa is undergoing a transformative shift in pharmaceutical and biopharmaceutical manufacturing, moving from near-complete import dependency toward regional self-sufficiency. With Member States importing between 70% and 100% of finished pharmaceutical products and 99% of vaccines, the continent faces both significant challenges and unprecedented opportunities. The African Continental Free Trade Area (AfCFTA) will create a continental market with a population of about 1.3 billion people and a combined GDP of approximately US$ 3.4 trillion, positioning Africa as an attractive destination for pharmaceutical partnerships and market expansion. The goal of the Partnerships for African Vaccine Manufacturing (PAVM) is to enable the African vaccine manufacturing industry to develop, produce, and supply over 60 per cent of the total vaccine doses required on the continent by 2040, up from less than 1 per cent today, with interim goals of 10 per cent by 2025 and 30 per cent by 2030.
The Current Pharmaceutical Landscape
Market Reality and Import Dependency
Ninety-five per cent of all medicines used in Africa are imported and the continent accounts for just 3% of all medicine production globally. This heavy reliance on external suppliers creates substantial vulnerabilities in Africa's health security infrastructure.
The import dependency extends across multiple product categories:
Finished Pharmaceutical Products: Member States of the WHO African Region import between 70% and 100% of finished pharmaceutical products
Vaccines: 99% of vaccines used across the continent are imported
Medical Devices and APIs: Between 90% and 100% of medical devices and active pharmaceutical ingredients (APIs) come from external sources
Vaccine Production Capacity: Of the 1% (12 million doses) produced domestically, most are relegated to the final fill-and-finish steps
Manufacturing Infrastructure
A recent survey by Africa CDC on Africa's manufacturing landscape identified 574 manufacturers across the continent, including 25 dedicated to vaccine production – 10 of which already have installed capacity. This represents the foundation upon which Africa's pharmaceutical ambitions are being built.
Strategic Initiatives Driving Transformation
The African Continental Free Trade Area (AfCFTA)
Regional value chains are being developed, under the AfCFTA Private Sector Engagement Strategy, to offer African countries an opportunity to use regional advantages to boost competitiveness, diversify product supply, and export products with higher value-addition. The strategy focuses on four initial priority sectors or value chains, namely agroprocessing, automotive, pharmaceuticals, and transportation and logistics, based on the potential for import substitution and existing production capabilities on the continent.
The AfCFTA's pharmaceutical focus reflects the sector's strategic importance for both health security and economic development across the continent.
Partnerships for African Vaccine Manufacturing (PAVM)
In 2021, the African Union Heads of States and Governments established The Partnerships for African Vaccine Manufacturing (PAVM) under the Africa CDC. This initiative represents Africa's most ambitious effort to achieve vaccine sovereignty.
The Framework for Action sets forth the key diagnostic findings on the current vaccine manufacturing environment in Africa and recommends eight bold programs to unlock Africa's potential to grow and scale vaccine development and manufacturing over the next two decades.
Financial Commitments and Investment Mechanisms
African Vaccine Manufacturing Accelerator (AVMA)
Gavi, the Vaccine Alliance, launched its replenishment campaign asking for $9 billion in new pledges, out of a total need of $11.9 billion, with $2.9 billion available in existing donor pledges, investment income, and leftover resources from the COVID-19 pandemic.
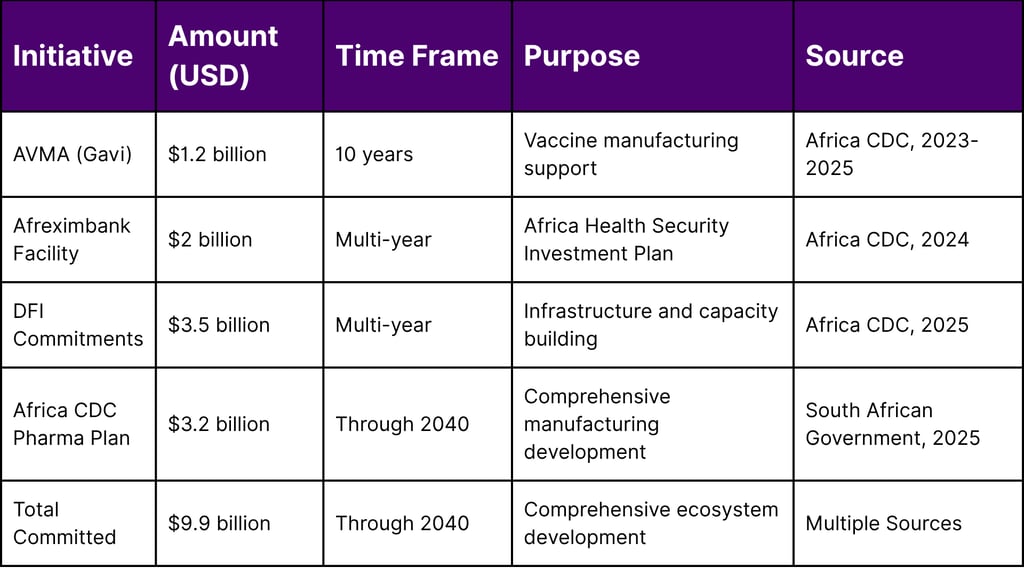
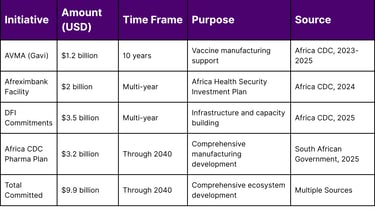
Gavi's African Vaccine Manufacturing Accelerator (AVMA) has committed US$1.2 billion over 10 years to support sustainable vaccine production across Africa.
Additional Financing Mechanisms
The Africa CDC and African Export-Import Bank (Afreximbank) renewed their partnership with a new cooperation agreement announced on 20 June. Through this collaboration, Afreximbank committed a USD 2 billion facility to the "Africa Health Security Investment Plan" to support the health product manufacturing ambition of the continent.
Over US$3.5 billion in commitments from global donors and development finance institutions, including the European Investment Bank, the International Finance Corporation and the US Development Finance Corporation have been secured.
The plan, initiated in 2025, is supported by a significant investment of US$3.2 billion, demonstrating unprecedented financial backing for Africa's pharmaceutical transformation.
Table 1: Major Financial Commitments to African Pharmaceutical Manufacturing
Workforce Development Initiatives
Between 2025 and 2030, three African vaccine manufacturers are expected to produce and secure World Health Organization (WHO) Prequalification for eight vaccines to supply the continental market and beyond.
Scaling vaccine manufacturing and R&D across the continent to meet PAVM's 2040 ambitions will require quadrupling the vaccine workforce to approximately 12,500 full-time employees (FTEs). Currently, there is a scarcity of vaccine development and manufacturing talent in Africa. Today, this talent totals between 2,000 and 3,000 FTEs, of whom many are associated with R&D entities that are not fully vaccine dedicated.
Vaccine Manufacturing Workforce Gap
Current Workforce: 2,000-3,000 FTEs
Required by 2040: 12,500 FTEs
Gap to Close: 9,500-10,500 FTEs
Growth Required: 4x current capacity
The Africa Centres for Disease Control and Prevention (Africa CDC) has established Regional Capability and Capacity Networks (RCCNs) to drive skills development, workforce training and R&D.
Emerging Manufacturing Hubs
South Africa: Continental Leader
South Africa has positioned itself as the continent's pharmaceutical manufacturing powerhouse with the most advanced infrastructure and regulatory environment.
As the Government of South Africa, we have consciously placed science, technology, and innovation at the heart of our country's development strategy. Through our Department of Science, Technology and Innovation (DSTI), our actions are guided by the Decadal Plan on Science, Technology and Innovation (2022-2032).
The Decadal Plan specifically identifies Health Innovation as a core STI priority, alongside energy innovation, and explicitly targets the development of domestic capabilities across the entire health value chain- from discovery to local manufacturing of vaccines, active pharmaceutical ingredients.
API Manufacturing Initiatives
Previous attempts to establish local API manufacturing by attracting multinationals with these capabilities have not been successful. South Africa is the largest procurer of ARVs in the world (by volume) and sadly, 100% of the APIs used to make ARVs are imported.
South Africa currently spends approximately R15 billion a year on imported APIs, and the cluster is expected to help reduce these costs significantly. The API Technology Innovation Cluster is an initiative of the Department of Science and Innovation, the Technology Innovation Agency (an entity of the Department), and North-West University.
Egypt: Regulatory Excellence
Egypt has emerged as Africa's regulatory leader, achieving unprecedented milestones in medicines regulation.
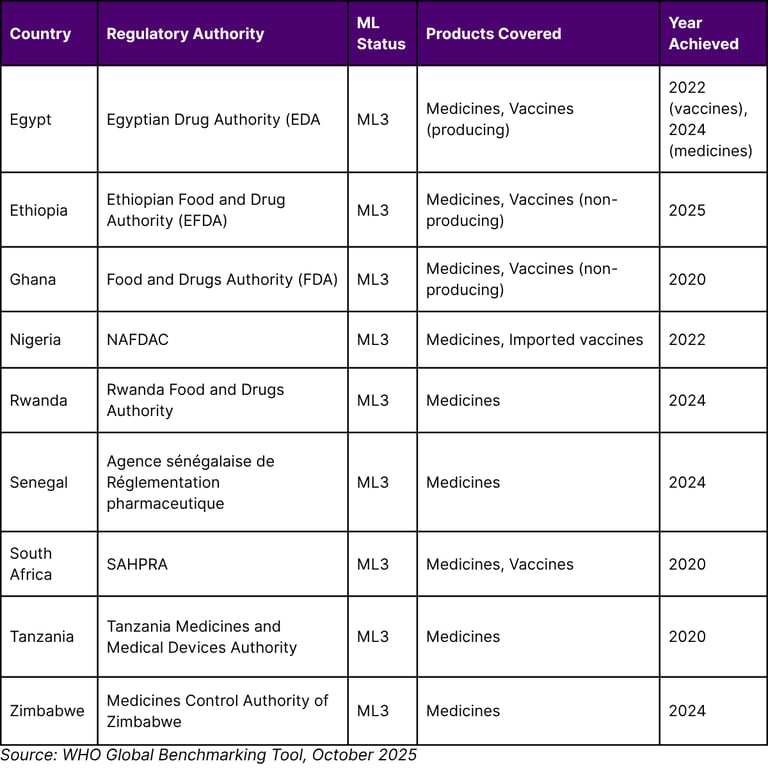
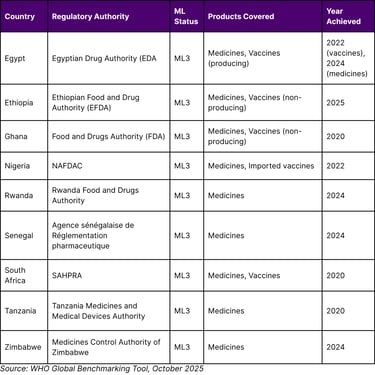
Egypt has achieved a significant milestone in medicines regulation, attaining maturity level 3 (ML3) in the World Health Organization's (WHO) global classification of national regulatory authorities. With this latest recognition, Egypt becomes the first country in Africa to achieve ML3 for both medicines and vaccines regulation, as assessed by WHO's Global Benchmarking Tool (GBT).
This ML3 designation follows a formal benchmarking process conducted by WHO, marking significant progress toward strengthening the regulatory system for medical products across the African continent. Notably, this achievement builds on Egypt's earlier success in March 2022, when it reached ML3 for vaccines regulation (locally produced and imported).
In December 2024, the World Health Organization recognised it as the first African country to achieve Maturity Level 3 for medicines and vaccines – a milestone that signals a well-regulated and stable pharmaceutical sector. This achievement coincided with Egypt's production of its first locally manufactured insulin.
Kenya: First WHO-Prequalified African ARV Manufacturer
In 2023, Universal Corporation Ltd (UCL), a Kenya-based pharmaceutical company led by Mr Palu Dhanani, became the first African manufacturer to receive WHO prequalification to produce tenofovir disoproxil fumarate, lamivudine and dolutegravir (TLD), a WHO-recommended first-line antiretroviral therapy for HIV infection.
As recently announced, the Global Fund now procures UCL's TLD for Mozambique, marking the first time TLD is manufactured on African soil. This milestone reflects ongoing collaboration between WHO and the Global Fund to support essential HIV services, through the NextGen market shaping approach.
Senegal: New Vaccine Production Hub
Senegal's leadership in this space is widely recognised, especially with the establishment of the MADIBA vaccine production hub, inaugurated in December 2024.
Senegal and Rwanda have become the seventh and eighth countries in Africa to reach Maturity Level 3 (ML3) in WHO's global classification of national regulatory authorities, underscoring their commitment to ensuring safe, effective and high-quality medical products for their populations.
Ethiopia: Strategic Manufacturing Development
The national strategy is aligned and harmonized with the next five years' Health Sector Transformation Plan and the Growth and Transformation Plan (GTP II) to enhance the capacity of manufacturers towards ensuring supply of affordable and quality medicines to the local and regional markets, including the competitive international market.
Ethiopia is one of the fastest growing economies in the world, with an average growth of around 10.9% for the past decade. There is a national aspiration to graduate to middle income country status by 2020–2025.
Table 2: African Countries with WHO Maturity Level 3 Regulatory Status (as of October 2025)
Regulatory Harmonization and Quality Assurance
African Medicines Agency (AMA)
Currently, thirty (64%) Member States have signed, ratified and deposited the AMA ratification instruments with the AU commission. The WHO African Regional Office has seconded two employees to support the operationalization of AMA.
WHO supported the African Union Commission (AUC) in the deployment of a special envoy to engage African Heads of State and advocate for the signing of the African Medicines Agency (AMA) Treaty resulting in its establishment and entry into force after the required 15 ratifications.
Thirty (30) African Union (AU) Member States have now completed the AMA ratification processes (Algeria, Benin, Botswana, Burkina Faso, Cabo Verde, Cameroon, Chad, Côte d'Ivoire, Egypt, Ethiopia, Gabon, Ghana, Guinea, Kenya, Lesotho, Mali, Mauritius, Morocco, Namibia, Niger, Rwanda, Sahrawi Arab Democratic Republic, Senegal, Seychelles, Sierra Leone, Tunisia, Uganda, United Republic of Tanzania, Zambia and Zimbabwe).
African Vaccine Regulatory Forum (AVAREF)
WHO supported the expansion of medicines regulation in subregional economic communities from two subregions in 2016 to all six subregions in 2025.
AVAREF has reduced the turnaround time for assessing clinical trials from between 6 months and 1 year to 30 days for expedited applications and 10 to 15 days for emergency applications.
Demand Creation and Procurement Mechanisms
African Pooled Procurement Mechanism (APPM)
Establish a clear and mutually agreed mechanism for Member States to express their preference for African-made vaccines and other health products, leveraging existing and future procurement systems. Furthermore, urge international procurers to COMMIT to procure at least 30% of their purchases for Africa from Africa.
Member states and manufacturers will benefit from committing parts of healthcare product demand volumes to Africa CDC for consolidated procurement through the APPM. This will ensure better pricing and reliable supply while strengthening local production capacity and reducing import dependence for pharmaceuticals, diagnostics, and other critical medical products.
Projected Vaccine Market Entry
The African Vaccine Manufacturing Landscape; a report prepared by Africa CDC, CHAI and PATH projects 8 Antigens to achieve WHO prequalification (PQ) and enter the continental market between 2025 – 2030. Ensuring demand certainty and predictability through offtake agreements of the near-term African Vaccine Manufacturer's products is crucial for the sustainability of vaccine manufacturing in Africa.
Timeline for African Vaccine Manufacturing Milestones
2021: PAVM Established
└─ Target: 60% local production by 2040
2023: AVMA Announced
└─ $1.2B committed over 10 years
2024: MADIBA Hub Inaugurated (Senegal)
└─ Egypt achieves ML3 for medicines
2025: 3 Manufacturers ramping up
└─ Target: 10% local production
└─ 8 vaccines projected for WHO PQ
2025-2030: 8 Vaccines to achieve WHO Prequalification
└─ Continental market entry
2030: Target 30% local production
2040: Target 60% local production
└─ 12,500 FTEs employed
Opportunities for International Partnerships
Technology Transfer and Manufacturing Hubs
Technical cooperation with Germany, the European Union, and Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) -- through initiatives such as SAVax Joint Action and the Team Europe Initiative MAV+ -- is bringing targeted support specifically geared towards establishing robust mRNA technology platforms. This includes providing good manufacturing practice (GMP) support, regulatory strengthening and essential business development expertise.
WHO supported the AUC in the deployment of a special envoy to engage African Heads of State and advocate for the signing of the AMA Treaty resulting in the establishment of the AMA after the required ratification by 15 countries was achieved.
Market Access Through AfCFTA
African market integration and trade facilitation is crucial and African countries must make better use of regional economic integration platforms such as the Economic Community of West African States, the Common Market for Eastern and Southern Africa and The new African Continental Free Trade Agreement offer great opportunities. More integration will help lead to the manufacturing of products that are in high demand in the region, will expand access to more markets and make local production sustainable.
The fourth Intra-African Trade Fair (IATF2025), hosted in Algiers, Algeria, concluded on 10 September 2025 with resounding success, recording US$48.3 billion in trade and investment deals signed during the weeklong continental exposition.
Research and Development Collaboration
Countries should formulate education policies that foster research and development in pharmaceuticals as well as encouraging thousands more people to gain skills to thrive in the industry. Countries must strengthen and harmonize their regulatory systems to ensure all medical products are of the highest quality and that local manufacturers adhere to international standards.
Challenges and Risk Mitigation
Current Bottlenecks
Fragmented regulatory systems, limited technology, workforce shortages, lack of off-take guarantees, and gaps in financing have hindered regional manufacturing growth.
Dr Kaluwa underscored that reliance on imported medicines continues to pose significant health security risks for African countries, particularly in times of global supply chain disruptions.
Addressing the Skills Gap
Dr Chiluba Mwila, talent development lead for Africa CDC's Platform for Harmonised African Health Manufacturing (PHAHM), emphasised the need for industry-academia collaboration to develop STEM curricula, internships, and on-the-job training.
The two-day hands-on workshop, held from 22–23 July 2025, brought together 26 participants from National Regulatory Authorities (NRAs) and pharmaceutical manufacturers across seven East African Community (EAC) member states. It was jointly organized by the Local Production and Assistance Unit in WHO Headquarters, the WHO Regional Office for Africa (AFRO), and the WHO Country Office in Ethiopia, with financial support from The Global Fund to Fight AIDS, Tuberculosis and Malaria.
Future Outlook and Strategic Priorities
Continental Vision
Africa currently produces less than 1% of the vaccines it consumes, leaving the region vulnerable to supply chain disruptions and global inequities. The health leaders committed to increase domestic investment, expand clinical trial capacity and implement the Regional Framework for Strengthening Local Production of Medicines, Vaccines, and Other Health Technologies (2025–2035).
"The issue of local manufacturing must be high on Africa's agenda," said H.E Hakainde Hichilema, President of the Republic of Zambia at the opening of the Regional Committee. "Zambia will take the lead in local products manufacturing, and we hope other countries can join to improve the well-being of people across the region."
"For Africa to produce 60% of its own vaccines by 2040," said Dr Mohamed Janabi, WHO Regional Director for Africa. "With stronger political will and smarter collaboration, we can improve Africa's ability to respond to the next pandemic—on our terms."
Strategic Recommendations for Market Entry
For pharmaceutical companies and investors considering Africa's biopharma sector:
Leverage AfCFTA Framework: Position operations to serve the continental market of 1.3 billion people rather than individual country markets
Focus on Priority Products: Align manufacturing plans with the 8 vaccines projected for WHO prequalification by 2030 and essential medicines with guaranteed demand
Engage with AVMA: Access the $1.2 billion financing mechanism designed specifically to support sustainable African vaccine manufacturing
Build Regulatory Capacity: Partner with countries achieving ML3 status to ensure faster market access and WHO prequalification eligibility
Invest in Workforce Development: Collaborate with Regional Capability and Capacity Networks to build the skilled workforce required for sustainable operations
Secure Off-take Agreements: Engage with the African Pooled Procurement Mechanism to secure demand certainty
Table 3: Key Market Entry Considerations for International Partners
Conclusion
Africa's biopharma sector stands at an inflection point. With nearly US$50 million committed by Unitaid to two flagship programmes that will boost Africa's capacity to produce medical diagnostics and therapeutics, and partners ready to reshape markets and enable African institutions to thrive, the foundations for transformation are firmly in place.
Africa CDC Director General Dr Jean Kaseya reaffirmed the continent's vision: "The agenda of local manufacturing is not an option; it's a vision we are materialising".
For pharmaceutical companies, the message is clear: Africa is no longer merely a market to serve through imports, but an emerging manufacturing powerhouse offering strategic opportunities for partnership, innovation, and growth. The combination of massive financial commitments, regulatory harmonization, workforce development initiatives, and continental market integration creates a compelling value proposition for forward-thinking organizations ready to participate in Africa's pharmaceutical transformation.
Frequently Asked Questions (FAQ)
Q1: What is the current state of pharmaceutical manufacturing in Africa?
Member States of the WHO African Region import between 70% and 100% of finished pharmaceutical products, 99% of vaccines, and between 90% and 100% of medical devices and active pharmaceutical ingredients (APIs). A recent survey by Africa CDC identified 574 manufacturers across the continent, including 25 dedicated to vaccine production – 10 of which already have installed capacity.
Q2: What is the PAVM target for local vaccine production?
The goal of the Partnerships for African Vaccine Manufacturing (PAVM) is to enable the African vaccine manufacturing industry to develop, produce, and supply over 60 per cent of the total vaccine doses required on the continent by 2040, up from less than 1 per cent today, with interim goals of 10 per cent by 2025 and 30 per cent by 2030.
Q3: How much funding has been committed to African pharmaceutical manufacturing?
Multiple funding commitments have been secured: Gavi's African Vaccine Manufacturing Accelerator (AVMA) has committed US$1.2 billion over 10 years, Afreximbank committed a USD 2 billion facility to the "Africa Health Security Investment Plan", and over US$3.5 billion in commitments from global donors and development finance institutions.
Q4: Which African countries have achieved WHO Maturity Level 3 regulatory status?
Senegal and Rwanda have become the seventh and eighth countries in Africa to reach Maturity Level 3 (ML3). Other countries at ML3 in Africa are Egypt, Ghana, Nigeria, South Africa, Tanzania and Zimbabwe. As of October 2025, Ethiopia has also achieved ML3 status.
Q5: What is the African Continental Free Trade Area's role in pharmaceutical manufacturing?
The AfCFTA will create a continental market with a population of about 1.3 billion people, a combined GDP of approximately US$ 3.4 trillion, enable African based manufacturers to have economies of scale and compete with the larger pharmaceuticals companies that export into Africa. The strategy focuses on four initial priority sectors or value chains, namely agroprocessing, automotive, pharmaceuticals, and transportation and logistics.
Q6: What workforce development is needed to meet manufacturing targets?
Scaling vaccine manufacturing and R&D across the continent to meet PAVM's 2040 ambitions will require quadrupling the vaccine workforce to approximately 12,500 full-time employees (FTEs). Currently, there is a scarcity of vaccine development and manufacturing talent in Africa. Today, this talent totals between 2,000 and 3,000 FTEs.
Q7: Which African manufacturer first achieved WHO prequalification for ARVs?
In 2023, Universal Corporation Ltd (UCL), a Kenya-based pharmaceutical company, became the first African manufacturer to receive WHO prequalification to produce tenofovir disoproxil fumarate, lamivudine and dolutegravir (TLD), a WHO-recommended first-line antiretroviral therapy for HIV infection.
Q8: How many vaccines are expected to achieve WHO prequalification from African manufacturers?
Between 2025 and 2030, three African vaccine manufacturers are expected to produce and secure World Health Organization (WHO) Prequalification for eight vaccines to supply the continental market and beyond.
Q9: What is Egypt's significance in African pharmaceutical regulation?
Egypt becomes the first country in Africa to achieve ML3 for both medicines and vaccines regulation, as assessed by WHO's Global Benchmarking Tool (GBT). In December 2024, the World Health Organization recognised it as the first African country to achieve Maturity Level 3 for medicines and vaccines – a milestone that signals a well-regulated and stable pharmaceutical sector.
Q10: What is the African Medicines Agency and how many countries have joined?
The African Medicines Agency (AMA) is a continental regulatory body designed to harmonize pharmaceutical regulation across Africa. Currently, thirty (64%) Member States have signed, ratified and deposited the AMA ratification instruments with the AU commission.
References
All data in this article is sourced exclusively from official government and international organization websites:
World Health Organization (WHO)
WHO Regional Office for Africa. (2024). Framework for strengthening local production of medicines, vaccines and other health technologies in the WHO African Region. AFR/RC74/6.
WHO Regional Office for Africa. (2025). Progress Report Regional Strategy of Medical Products. AFR/RC75/INF.DOC/8.
WHO. (2022). Inside Africa's drive to boost medicines and vaccine manufacturing.
WHO. (2024). Egypt's regulatory system reaches WHO maturity level 3 in medicines regulation.
WHO. (2024). Senegal and Rwanda achieve WHO Maturity Level 3 in medicines regulation.
WHO. (2025). Redefining the HIV response in Africa through local production of medicines and diagnostics.
WHO. (2025). Africa Must Reduce Reliance on Imported Medicines, WHO Urges at Regional GMP Training Workshop.
WHO. (2015). Stakeholders Agree on the National Strategy and Plan of Action 2015-2025 for Pharmaceutical Manufacturing Development in Ethiopia.
WHO. (2025). Health leaders, partners pledge to catalyse Africa's production of medicines and vaccines.
WHO. (2025). List of National Regulatory Authorities (NRAs) operating at maturity level 3 and 4.
Africa Centres for Disease Control and Prevention (Africa CDC)
Africa CDC. (2025). Regional Networks to Strengthen Africa's Vaccine and Health Products Manufacturing Workforce.
Africa CDC. (2025). New Dawn for Health Security and Sovereignty in Africa as Stakeholders Convene at Manufacturing Forum.
Africa CDC. (2024). The African Vaccine Manufacturing Accelerator is a Boon for the Continent.
Africa CDC. (2023). A Breakthrough for the African Vaccine Manufacturing.
Africa CDC. (2025). Africa CDC and Partners Launch Critical Initiative to Strengthen Health Product Manufacturing Workforce.
Africa CDC. (2024). Africa CDC and Afreximbank renew partnership to boost health manufacturing and strengthen disease surveillance.
Africa CDC. (2023). Framework for Action – Partnerships for African Vaccine Manufacturing.
South African Government
Government of South Africa. (2025). Minister Blade Nzimande: Science, Technology and Innovation Budget Vote 2025/26.
Government of South Africa. (2024). Minister Blade Nzimande: API Technology Innovation Cluster Launch.
African Union
African Union. (2025). Intra-African Trade Fair 2025 concludes with $48.3 billion in trade and investment deals.
African Union. (2024). African Union Commission welcomes Senegal's inauguration of MADIBA vaccine production hub.
Unitaid
Unitaid. (2024). Unitaid commits nearly US$ 50 million to boost Africa's capacity to produce medical diagnostics and therapeutics.

