AI-Driven Drug Discovery in Oncology


The convergence of artificial intelligence and oncology drug discovery represents one of the most promising frontiers in modern healthcare. The integration of artificial intelligence (AI) into oncology drug discovery is redefining the traditional pipeline by accelerating discovery, optimizing drug efficacy, and minimizing toxicity. This comprehensive analysis examines current technological breakthroughs, regulatory developments, government investments, and future opportunities that define this rapidly evolving landscape.
Traditional drug discovery faces persistent challenges, including high attrition rates, billion-dollar costs, and timelines spanning over a decade. AI technologies are addressing these fundamental challenges by providing new approaches to target identification, drug design, and patient stratification in oncology applications.
Government Research and Development Initiatives
NIH and NCI Leadership in AI Oncology Research
The National Institutes of Health (NIH) and National Cancer Institute (NCI) have emerged as leading forces in AI-driven oncology research. In a proof-of-concept study, researchers at the National Institutes of Health (NIH) have developed an artificial intelligence (AI) tool that uses data from individual cells inside tumors to predict whether a person's cancer will respond to a specific drug.
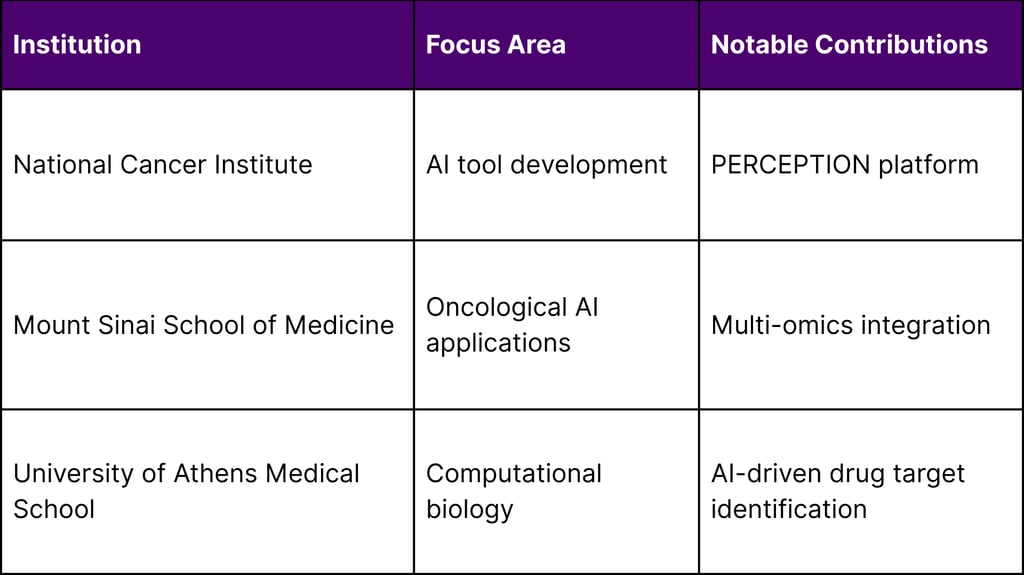
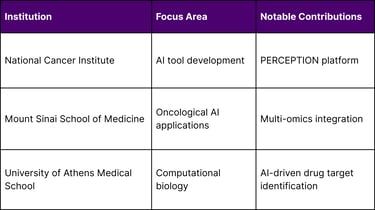
An AI tool called PERCEPTION developed by NCI researchers could one day be used to help more precisely match patients with effective cancer drugs, using data from individual cells inside tumors to predict whether a person's cancer will respond to a specific drug.
Federal AI Research Funding Landscape
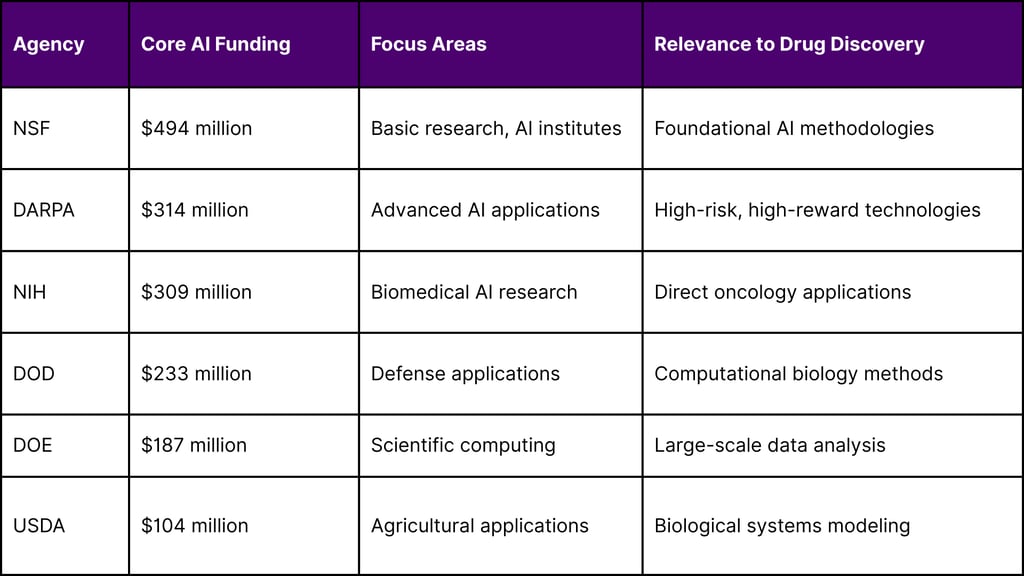
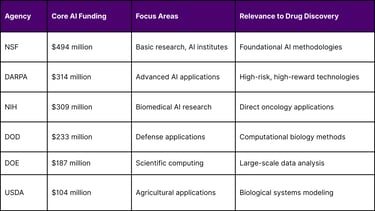
For core AI funding, the top agencies in order are NSF ($494 million), DARPA ($314 million), NIH ($309 million), DOD ($233 million), DOE ($187 million), and Agriculture ($104 million). This substantial federal investment demonstrates the government's commitment to advancing AI capabilities across scientific research domains.
The Department of Energy (DOE) announced $68 million in funding for 11 multi-institution projects, comprising 43 awards. The funded projects will develop new ways to create foundation models for scientific research.
Federal AI Research Investment Distribution (2024):
NIST AI Standards and Manufacturing Initiatives
NIST anticipates funding up to $70 million over a five-year period, subject to the availability of federal funds, for the recipient to establish and operate the new AI-focused Manufacturing USA institute. This initiative will support AI applications in manufacturing processes, including pharmaceutical manufacturing.
The U.S. Department of Commerce's National Institute of Standards and Technology (NIST) has awarded over $1.8 million to 18 small businesses under the Small Business Innovation Research (SBIR) program for research and development of new AI products.
Regulatory Framework Development
FDA AI Guidance for Drug Development
The CDER AI Council, established in 2024 to provide oversight, coordination, and consolidation of CDER activities around AI use, represents the FDA's commitment to developing comprehensive AI guidance for drug development.
The FDA's draft guidance discusses the use of AI models in the nonclinical, clinical, postmarketing, and manufacturing phases of the drug product life cycle, where the specific use of the AI model is to produce information or data to support regulatory decision-making regarding safety, effectiveness.
The FDA guidance provides a risk-based credibility assessment framework that may be used for establishing and evaluating the credibility of an AI model for a particular context of use (COU).
Regulatory Timeline and Implementation
The US Food and Drug Administration (FDA) plans to release a draft guidance this year on the use of artificial intelligence/machine learning (AI/ML) to support drug development, informed by the agency's experience in reviewing submissions containing AI/ML elements.
Technology Applications and Breakthroughs
Single-Cell Analysis and Precision Medicine
Current approaches to matching patients to drugs rely on bulk sequencing of tumor DNA and RNA, which takes an average of all the cells in a tumor sample. However, tumors contain more than one type of cell and in fact can have many different subpopulations of cells, known as clones.
A newer technology known as single-cell RNA sequencing provides much higher resolution data, down to the single-cell level. Using this approach to identify and target individual clones may lead to more lasting drug responses.
AI-Powered Drug Response Prediction
The researchers investigated whether they could use a machine learning technique called transfer learning to train an AI model to predict drug responses using widely available bulk RNA sequencing data, but then fine-tune that model using single-cell RNA sequencing data. Using this approach on published cell-line data from large-scale drug screens, the researchers built AI models for 44 Food and Drug Administration–approved cancer drugs.
Clinical Validation Results
The researchers then tested their approach on published data for 41 patients with multiple myeloma treated with a combination of four drugs and 33 patients with breast cancer treated with a combination of two drugs. The researchers discovered that if just one clone were resistant to a particular drug, the patient would not respond to that drug, even if all the other clones responded.
Academic Research and Publications
Recent Academic Breakthroughs
Artificial intelligence (AI) encompasses a broad spectrum of techniques that have been utilized by pharmaceutical companies for decades, including machine learning, deep learning, and other advanced computational methods.
Recent research published in Cancers (Basel) in October 2024 explores the fusion of artificial intelligence and machine learning for anti-cancer drug discovery, highlighting advances from leading academic institutions.
Academic-Industry Collaboration Models
Academic institutions are increasingly partnering with pharmaceutical companies to advance AI drug discovery capabilities. This work was conducted by NCI's Center for Cancer Research and led by Alejandro Schaffer, Ph.D., and Sanju Sinha, Ph.D., previously at NCI, now at Sanford Burnham Prebys.
Key Academic Research Centers:
Market Opportunities and Growth Drivers
Technology-Driven Market Expansion
In 2024, the use of AI in drug discovery has expanded, but has not yet reached its full potential. Technological and ethical challenges remain, but experts are on their way to solving these problems, and investment in the field is continuing to grow in response.
Primary Growth Catalysts
Regulatory Clarity and Support The development of comprehensive FDA guidance and establishment of the CDER AI Council provides the regulatory framework necessary for commercial development and approval of AI-discovered drugs.
Federal Research Investment Substantial federal funding across multiple agencies creates a foundation for continued technological advancement and academic-industry collaboration.
Clinical Validation Success Demonstrated success in clinical applications, such as the PERCEPTION platform's validation across multiple cancer types, provides proof of concept for commercial applications.
Cost and Timeline Benefits AI addresses traditional drug discovery challenges, including high attrition rates, billion-dollar costs, and lengthy development timelines.
Emerging Market Segments
Precision Oncology Applications Single-cell RNA sequencing data could one day be used to help doctors more precisely match cancer patients with drugs that will be effective for their cancer.
Combination Therapy Optimization The AI models accurately predicted how individual cells would respond to both single drugs and combinations of drugs.
Resistance Prediction and Management The AI model successfully predicted the development of resistance in published data from 24 patients treated with targeted therapies for non-small cell lung cancer.
Investment Landscape Analysis
Government Investment Patterns
Federal investment in AI research provides a foundation for private sector development. The concentration of funding across NSF, DARPA, NIH, DOD, and DOE creates a comprehensive research ecosystem supporting AI drug discovery applications.
Academic Commercialization Opportunities
The researchers have developed a research website and a guide for how to use the AI model, called Personalized Single-Cell Expression-based Planning for Treatments In Oncology (PERCEPTION), with new datasets. This represents the type of academic technology transfer that creates commercial opportunities.
Strategic Partnership Models
The movement of researchers between academic institutions and private companies, as demonstrated by the transition from NCI to Sanford Burnham Prebys, illustrates the dynamic nature of talent and technology transfer in this sector.
Challenges and Risk Factors
Technical Challenges
Data Availability and Quality Single-cell gene expression data are much more costly to generate than bulk gene expression data and not yet widely available in clinical settings.
Model Accuracy and Validation The researchers cautioned that the accuracy of this technique will improve if single-cell RNA sequencing data become more widely available.
Regulatory and Implementation Challenges
Regulatory Framework Evolution While the FDA has established the CDER AI Council and developed draft guidance, the regulatory pathway for AI-discovered drugs continues to evolve, creating uncertainty for commercial development.
Clinical Integration The translation of academic research tools into clinical practice requires substantial validation and integration with existing healthcare systems.
Future Outlook Through 2030
Technology Evolution Predictions
Advanced AI Architectures Future developments will likely focus on improving model accuracy through expanded datasets and more sophisticated analytical approaches.
Integration with Clinical Workflows The accuracy of this technique will improve if single-cell RNA sequencing data become more widely available, suggesting that clinical adoption will drive continued improvement in AI capabilities.
Expanded Therapeutic Applications Success in multiple myeloma, breast cancer, and non-small cell lung cancer demonstrates the potential for expansion to additional cancer types.
Market Structure Evolution
Academic-Industry Integration The established pattern of researcher movement between academic institutions and industry suggests continued collaboration and technology transfer.
Federal Support Continuation Substantial federal investment across multiple agencies indicates sustained government support for AI research and development.
Regulatory Maturation The establishment of formal FDA guidance and oversight structures suggests increasing regulatory clarity and support for AI drug discovery applications.
Regional Analysis
United States Leadership
The concentration of federal funding, regulatory development, and academic research positions the United States as the global leader in AI-driven oncology drug discovery.
Academic Excellence Centers
Leading research institutions, including the National Cancer Institute, Mount Sinai, and other academic medical centers, provide the foundation for continued technological advancement.
Federal Research Infrastructure
The coordinated investment across NSF, NIH, DARPA, DOE, and other federal agencies creates a comprehensive research ecosystem supporting AI drug discovery development.
Investment Recommendations and Strategic Considerations
For Institutional Investors
Technology Validation Focus Investors should prioritize companies with demonstrated clinical validation, similar to the PERCEPTION platform's success across multiple cancer types.
Regulatory Alignment Companies aligned with FDA guidance and regulatory requirements are positioned for more predictable approval pathways.
Academic Partnership Strength Organizations with strong academic partnerships can leverage federal research funding and academic expertise.
For Pharmaceutical Companies
Academic Collaboration Strategies Partnerships with leading academic research centers provide access to cutting-edge technology and federal research funding.
Regulatory Preparation Early engagement with FDA guidance and CDER AI Council requirements can streamline approval processes.
Technology Integration Planning Successful implementation requires comprehensive planning for clinical workflow integration and data infrastructure development.
FAQ
Q1: What role does the government play in AI-driven oncology drug discovery?
The federal government plays a crucial leadership role through substantial research funding ($309 million from NIH alone), regulatory guidance development through the FDA's CDER AI Council, and comprehensive research infrastructure across multiple agencies.
Q2: What are the main AI technologies being used in oncology drug discovery?
Key technologies include single-cell RNA sequencing analysis, machine learning for drug response prediction, transfer learning techniques, and AI models for combination therapy optimization.
Q3: How effective are current AI tools in predicting cancer drug responses?
AI models built using transfer learning approaches accurately predicted how individual cells would respond to both single drugs and combinations of drugs, and successfully predicted the development of resistance in clinical datasets.
Q4: What regulatory framework governs AI-discovered drugs?
The FDA has developed a risk-based credibility assessment framework for AI models and draft guidance covering AI use in nonclinical, clinical, postmarketing, and manufacturing phases of drug development.
Q5: What are the main challenges facing AI drug discovery in oncology?
Primary challenges include the cost and limited availability of high-quality single-cell data, regulatory framework evolution, model validation requirements, and clinical workflow integration.
Q6: How much federal funding supports AI drug discovery research?
Core AI funding includes NSF ($494 million), DARPA ($314 million), NIH ($309 million), DOD ($233 million), DOE ($187 million), and USDA ($104 million).
Q7: What types of cancer show the most promise for AI applications?
Current validated applications include multiple myeloma, breast cancer, and non-small cell lung cancer, with demonstrated success in predicting drug responses and resistance development.
Q8: How do AI drug discovery timelines compare to traditional approaches?
While specific timeline data varies, AI technologies address traditional challenges of high attrition rates, high costs, and lengthy development periods by improving prediction accuracy and reducing trial-and-error approaches.
Q9: What should investors look for when evaluating AI drug discovery companies?
Key factors include clinical validation data, alignment with FDA regulatory guidance, strength of academic partnerships, quality of scientific teams, and demonstrated success in predicting drug responses.
Q10: What is the outlook for AI in oncology drug discovery through 2030?
The use of AI in drug discovery has expanded but has not yet reached its full potential, with continued growth expected as technological and ethical challenges are addressed.
References
National Cancer Institute. (2024). "AI tool helps predicts patient responses to cancer drugs."
National Institutes of Health. (2024). "NIH researchers develop AI tool with potential to more precisely match cancer drugs to patients."
National Institutes of Health. (2024). "NIH scientists develop AI tool to predict how cancer patients will respond to immunotherapy."
PMC. (2024). "Artificial Intelligence-Driven Innovations in Oncology Drug Discovery: Transforming Traditional Pipelines and Enhancing Drug Design."
PMC. (2024). "Applications of Artificial Intelligence in Biotech Drug Discovery and Product Development."
PMC. (2024). "Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine."
PMC. (2024). "Fusing Artificial Intelligence and Machine Learning for Anti-Cancer Drug Discovery." Cancers (Basel).
FDA. (2024). "Considerations for the Use of Artificial Intelligence To Support Regulatory Decision-Making for Drug and Biological Products."
FDA. (2024). "Artificial Intelligence for Drug Development."
Federal Register. (2025). "Considerations for the Use of Artificial Intelligence To Support Regulatory Decision-Making for Drug and Biological Products."
Department of Energy. (2024). "Department of Energy Announces $68 Million in Funding for Artificial Intelligence for Scientific Research."
Federal Budget IQ. (2024). "Federal AI and IT Research and Development Spending Analysis."
NIST. (2024). "NIST Announces Funding Opportunity for AI-Focused Manufacturing USA Institute."
NIST. (2025). "NIST Awards Over $1.8 Million to Small Businesses Advancing AI, Semiconductors, Additive Manufacturing and More."

