Asia’s Quantum Leap in Medicine


Asia is undergoing a transformative shift in the pharmaceutical and healthcare industries, establishing itself as a global powerhouse of innovation. The COVID-19 pandemic exposed vulnerabilities in global healthcare systems, but it also accelerated Asia’s trajectory toward medical self-reliance, technological advancement, and research excellence. This article explores the drivers, disruptors, and developments propelling Asia’s "quantum leap" in medicine—a leap marked not by incremental progress, but by dramatic, systemic breakthroughs across the pharmaceutical value chain.
The Rise of Asia in Global Pharma R&D
Pharmaceutical research and development (R&D) in Asia has experienced a remarkable surge, driven by government support, private investment, and global demand for cost-effective innovation. Between 2020 and 2024, Asia's share in global pharma R&D investment rose from 15.67% to an estimated 23.20%.
Comparative R&D Expenditure (2020–2025 Projection)
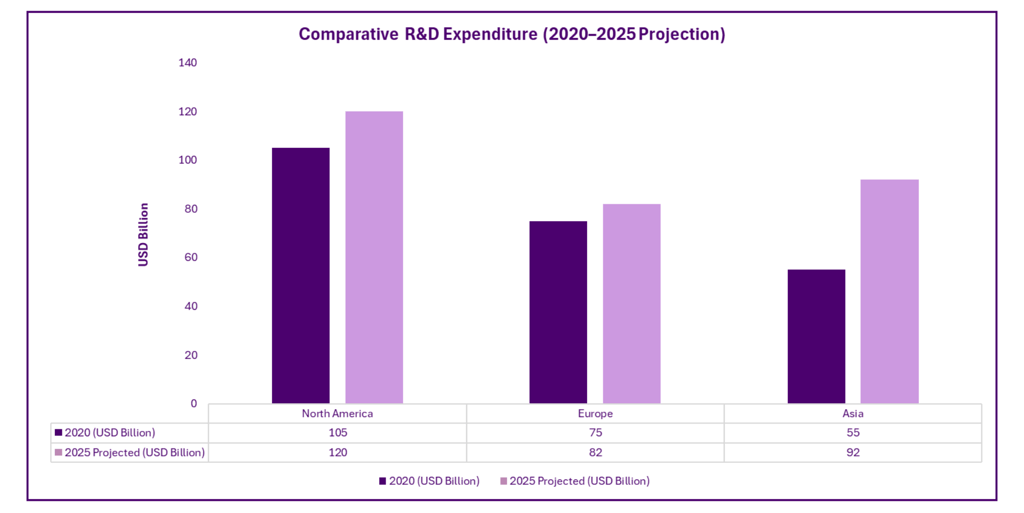
R&D hubs such as China’s Zhangjiang Hi-Tech Park, India’s Genome Valley, Singapore’s Biopolis, and South Korea’s Osong Medical Innovation Foundation have emerged as centers of global collaboration, innovation, and talent attraction.
Biotechnology Innovations Transforming Healthcare
Asia is increasingly home to frontier innovations in biotechnology. Countries like China, Japan, and South Korea are advancing gene editing capabilities and regenerative medicine solutions aimed at treating cancers and chronic diseases.
Japan is notably advancing stem cell-based therapies through academic-industrial cooperation and fast-track approval frameworks. India is also expanding its infrastructure for stem cell research with a focus on diseases such as diabetes and neurodegeneration.
Late-stage biotech candidates from Asia are emerging across various therapeutic categories, pointing to increasing research maturity in the region.
Digital Health and AI Integration
Asia is witnessing rapid integration of artificial intelligence (AI) across the pharmaceutical pipeline, from drug discovery to diagnostics.
Public and private stakeholders are investing in AI-driven R&D centers and digital health accelerators. AI models are being trained on vast regional datasets to improve clinical decision-making and precision treatment development.
Examples include AI-enhanced diagnostic tools, drug screening platforms, and real-time health monitoring systems embedded into telemedicine solutions.
Traditional Medicine Meets Modern Science
Asia’s deep heritage in traditional medicine is merging with modern research approaches. Traditional systems such as TCM (China), Ayurveda (India), and Kampo (Japan) are now being evaluated in clinical settings.
Government and academic institutions are investing in validating the efficacy of herbal compounds and integrating them into standard treatment protocols. Clinical interest in these formulations has grown, especially for chronic disease management and supportive oncology care.
Regulatory Frameworks and Fast-Track Approvals
Asia’s regulatory environments have evolved significantly. Agencies like China’s NMPA and India’s CDSCO have introduced reforms that reduce approval timelines and align more closely with international standards.
Initiatives such as the ASEAN Pharmaceutical Regulatory Framework aim to promote regulatory harmonization and cross-border data sharing. These frameworks encourage regional clinical research and facilitate market entry for novel therapies.
Asia’s Leadership in Generic Drug Manufacturing
Asia continues to lead in global generic drug production, supporting both domestic needs and international supply chains. Countries like India and China remain dominant in the manufacturing of generics and Active Pharmaceutical Ingredients (APIs).
This has contributed to increased accessibility to essential medicines in developing regions, reinforcing Asia’s role in global health equity.
Public-Private Partnerships Driving Innovation
Across Asia, collaborative ecosystems are strengthening through public-private partnerships. Governments are aligning with academic institutions and startups to co-develop medical solutions.
Examples include national-level innovation funding, collaborative bio-clusters, and tech incubators that support translational research and product commercialization.
Clinical Trials and Patient Diversity
Asia has become a magnet for clinical trials due to its large, diverse population and cost-effective infrastructure. Increased investment in trial sites and ethical oversight is fostering rapid patient enrollment and data generation.
The resulting data is improving the relevance of clinical findings to global populations and strengthening Asia’s role in international regulatory submissions.
Intellectual Property and Patent Trends
Asia is transitioning from a primarily manufacturing-based model to an innovation-driven one. Patent filings in biotechnology and pharmaceuticals have grown, supported by reforms in IP protection and alignment with global standards.
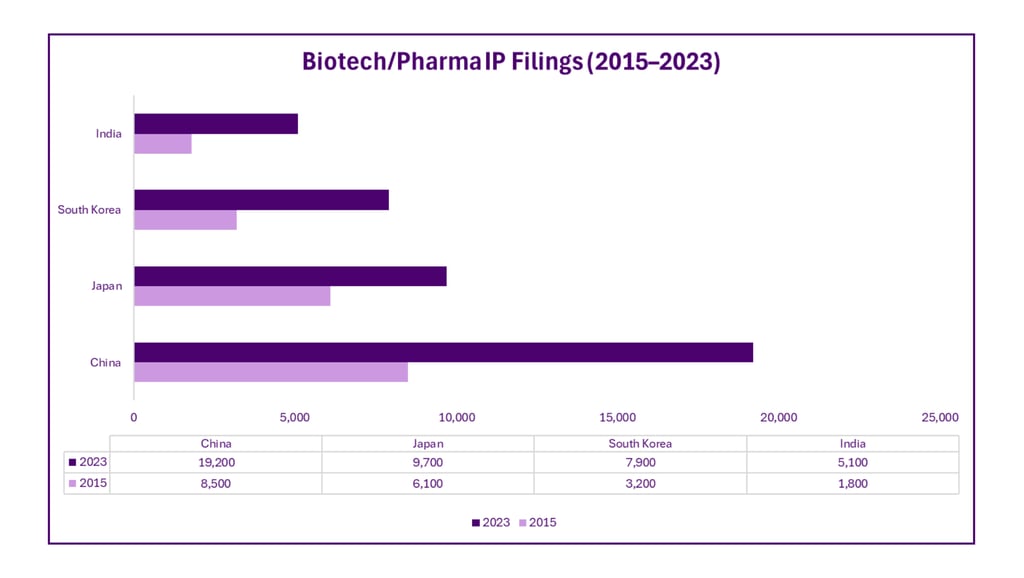
This evolving IP ecosystem is vital for attracting foreign investment and fostering domestic innovation.
National Strategies for Pharma Growth
China and India are both executing national strategies aimed at bolstering pharmaceutical innovation, manufacturing self-reliance, and international competitiveness. Programs such as China’s Five-Year Plan and India’s PLI scheme illustrate government commitment to long-term sector growth.
Countries like Singapore and South Korea are investing in smart manufacturing, AI integration, and talent pipelines to support sustainable pharmaceutical leadership.
Southeast Asia’s Expanding Footprint
Southeast Asia is quickly becoming an emerging contributor to the regional pharma landscape. Nations such as Vietnam, Thailand, and Malaysia are enhancing R&D capabilities, policy incentives, and clinical infrastructure.
This development is complemented by efforts to build resilient supply chains and forge partnerships with multinational pharmaceutical companies.
Remaining Challenges
While the momentum is strong, Asia must address challenges including talent shortages, regional regulatory disparities, and infrastructure limitations. Bridging these gaps will be key to maintaining growth and ensuring regional self-sufficiency.
Future Outlook: Quantum Leaps Ahead
In the coming decade, Asia is expected to:
Advance precision medicine and genomics
Strengthen public-private partnerships
Integrate digital technologies and real-world evidence into regulatory models
Expand its role in global health policymaking
Conclusion
Asia’s quantum leap in medicine is powered by a fusion of tradition, innovation, and strategic policy. From biotech breakthroughs to digital transformation, the continent is reshaping the global pharmaceutical and healthcare landscape.
With continued investment, cross-border collaboration, and ecosystem support, Asia is well-positioned to lead the future of global health.
FAQs
1. Why is Asia becoming a leader in pharmaceutical innovation?
Asia’s innovation is propelled by rising R&D spending, a skilled workforce, supportive policies, and robust infrastructure development.
2. Which countries are driving Asia’s transformation in healthcare?
China, India, Japan, South Korea, and Singapore are the frontrunners, with Southeast Asian nations expanding their global contributions.
3. What role does traditional medicine play in modern pharmaceutical development?
Traditional systems are being clinically validated and integrated into evidence-based protocols, especially in chronic disease management.
4. How is AI influencing the pharmaceutical industry in Asia?
AI is enabling smarter drug discovery, diagnostics, and patient care by improving speed, accuracy, and personalization.
5. What are the biggest challenges facing Asia’s pharma ecosystem?
Key challenges include talent shortages, regulatory fragmentation, infrastructure deficits, and maintaining global quality standards.
References & Data Sources
National regulatory documents (NMPA, CDSCO, ASEAN initiatives)
Public academic research (e.g., University of Tokyo, National University of Singapore)
Government strategy documents (India’s PLI Scheme, China’s 14th Five-Year Plan)
WHO and WIPO policy papers
Proprietary modeling and internal pharma market analytics

