ASO 2.0 Is Changing the Rules


Antisense oligonucleotide (ASO) therapies have entered a transformative phase marked by enhanced chemical modifications, improved delivery systems, and regulatory frameworks specifically designed for rare disease treatment. As of early 2026, the FDA has approved 17 RNA oligonucleotide therapeutics, including 11 antisense oligonucleotides targeting predominantly rare genetic disorders. With blood-brain barrier penetration breakthroughs and pediatric approvals on the horizon, ASO 2.0 represents a paradigm shift in precision medicine for ultra-rare conditions affecting millions globally.
Introduction: The Evolution of Antisense Technology
Since the first antisense oligonucleotide approval in 1998, the field has undergone remarkable evolution. The 2010s marked a turning point with breakthrough approvals for spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD), demonstrating that ASOs could effectively modify disease progression in previously untreatable conditions.
Today, ASO 2.0 encompasses:
Advanced chemical modifications including 2'-O-methoxyethyl (2'-MOE), phosphorothioate (PS) backbones, and phosphorodiamidate morpholino oligomers (PMO)
Next-generation delivery technologies utilizing GalNAc conjugation for hepatic targeting
Innovative blood-brain barrier penetration strategies
Streamlined regulatory pathways for individualized ASO development
The Rare Disease Landscape: A Growing Imperative
Epidemiological Overview
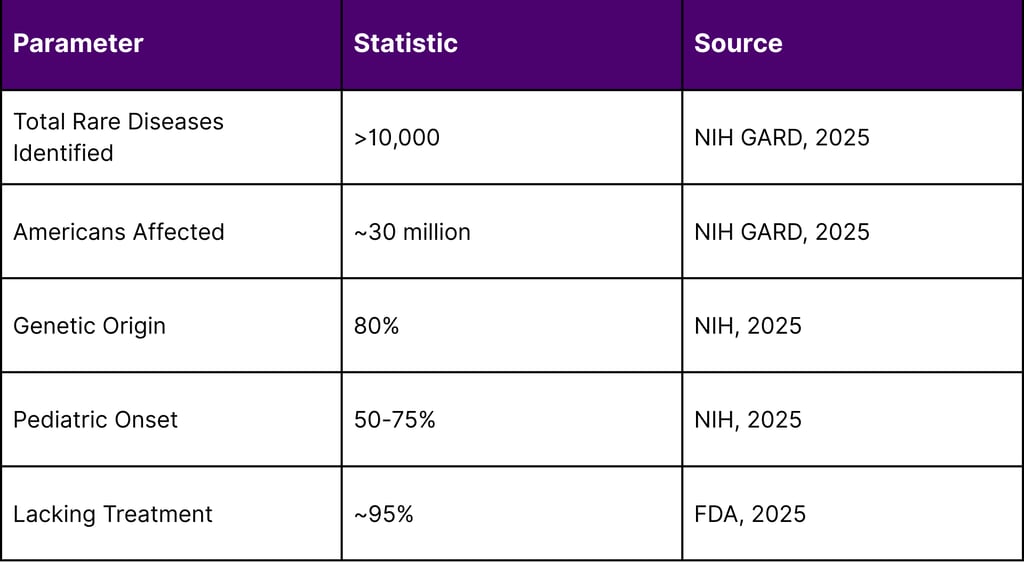
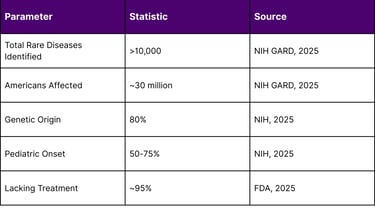
According to the National Institutes of Health (NIH) Genetic and Rare Diseases (GARD) Information Center, more than 10,000 rare diseases have been identified, affecting an estimated 30 million Americans. Key statistics reveal:
Genetic Origins: Approximately 80% of rare diseases have genetic origins
Pediatric Impact: 50-75% of rare diseases manifest in childhood
Diagnostic Delays: 25% of rare disease patients wait 5-30 years for accurate diagnosis
Treatment Gap: Approximately 95% of rare diseases lack FDA-approved treatments
This enormous unmet need creates both a humanitarian imperative and a substantial market opportunity for precision therapies like ASOs.
ASO 2.0: Key Technological Advancements
1. Enhanced Chemical Modifications
Modern ASO platforms leverage sophisticated chemical modifications to overcome the limitations of first-generation oligonucleotides. These modifications address three critical challenges: nuclease degradation, cellular uptake, and off-target effects.
Primary Chemical Strategies:
2'-O-Methoxyethyl (2'-MOE): Increases binding affinity and nuclease resistance
Phosphorothioate (PS) Backbones: Enhances protein binding and tissue distribution
Locked Nucleic Acids (LNA): Improves target specificity and reduces required dosage
Phosphorodiamidate Morpholino Oligomers (PMO): Provides charge-neutral backbone with enhanced safety profile
2. Blood-Brain Barrier Penetration: A Game Changer
Historically, CNS delivery required invasive intrathecal administration. Breakthrough research in 2024-2025 has demonstrated systemic CNS delivery capabilities:
Emerging BBB Strategies:
ApoE-Derived Peptide Conjugates: Research published in 2024 showed BPP8-PMO conjugates achieved 78% brain parenchyma penetration with 11% neuronal uptake following intravenous administration
Transferrin Receptor Targeting: Oligonucleotide transfer vectors (OTV) utilizing TfR1 have demonstrated uniform CNS distribution in primate models
Glucose Transporter-Mediated Delivery: Glucose-coated polymeric nanocarriers showed significant target RNA knockdown in cerebral cortex and hippocampus
These advances could eliminate the need for invasive lumbar punctures, dramatically improving patient compliance and expanding treatment accessibility.
3. GalNAc Conjugation for Hepatic Targeting
N-acetylgalactosamine (GalNAc) conjugation has revolutionized hepatic ASO delivery. The asialoglycoprotein receptor (ASGPR) expressed on hepatocytes facilitates rapid and efficient uptake, enabling:
Subcutaneous administration
Reduced dosing frequency (every 3-6 months)
Lower systemic exposure with enhanced target tissue concentration
Improved safety profiles
Recent FDA Approvals and Pipeline Analysis
2025 ASO Approvals
The FDA approved two significant antisense oligonucleotides in 2025:
Dawnzera (donidalorsen) - NDA 219407
Indication: Prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 12 years and older
Mechanism: Targets prekallikrein mRNA using LICA technology
Dosing: 80 mg subcutaneously every 4 weeks
PDUFA Date: August 21, 2025
Clinical Outcomes: Demonstrated significant and sustained reduction in mean monthly HAE attack rates
Tryngolza (olezarsen) - Approved December 19, 2024
Indication: Familial chylomicronemia syndrome (FCS)
Classification: First-in-class APOC-III-directed antisense oligonucleotide
Status: Marks breakthrough for small nucleic acid drugs in rare disease treatment
2026 Expected Approvals
According to FDA documentation and pharmaceutical company announcements:
DTX401 (gene therapy) and Multiple ASO Candidates Expected:
GTX-102 (apazunersen): Phase 3 pivotal data for Angelman syndrome expected in second half of 2026
Pelacarsen: Phase III data expected in first half of 2026, with regulatory submissions in second half of 2026
UX111: BLA resubmission planned for early 2026
Regulatory Innovation: Platform Technology Designation
The FDA's Platform Technology Designation program, introduced in 2023, provides expedited development pathways for well-characterized ASO platforms adapted to new targets. This regulatory innovation recognizes that oligonucleotide safety profiles depend primarily on chemical structure rather than target sequence.
Individualized ASO Guidance
The FDA published comprehensive guidance documents for individualized ASO drug products for severely debilitating or life-threatening diseases, covering:
Chemistry, Manufacturing, and Controls (CMC) recommendations
Clinical recommendations
Nonclinical testing requirements
Administrative and procedural recommendations
This framework enables "n-of-1" medicine, where ASOs can be custom-designed for ultra-rare genetic variants affecting single patients or small cohorts.
Pediatric Rare Disease Focus
Rare Pediatric Disease Priority Review Voucher Program
The Rare Pediatric Disease Priority Review Voucher (RPD PRV) program has been instrumental in accelerating ASO development. According to the National Organization for Rare Disorders (NORD):
63 vouchers granted since program creation in 2012
Treatments developed for 47 rare pediatric diseases
43 of these diseases previously had no treatment options
4 vouchers granted in 2025 alone for severe conditions
However, with FDA authority to grant rare pediatric designations having expired in 2024, and authority to grant priority review vouchers expiring on September 30, 2026, reauthorization is critical for continued innovation.
Pediatric-Onset Rare Disease Pipeline
Research published in 2025 projects approximately 45 new product approvals for pediatric-onset rare diseases by 2033, representing 14% growth in annual treated patients. Despite this progress, 95% of pediatric-onset rare diseases are projected to still have no approved treatments in the next decade.
Market Access and Reimbursement Considerations
Pricing Landscape
Rare disease therapies face unique pricing challenges due to:
Small patient populations requiring high unit prices for R&D recovery
High manufacturing complexity for individualized therapies
Lifelong treatment requirements for many conditions
Projected Market Impact: According to federal data on pediatric rare disease pipeline, incremental list price revenues are projected to increase from $28.2 billion in 2023 to $38.9 billion in 2033, representing $10.7 billion in incremental growth.
Value-Based Contracting
Emerging reimbursement models for ASO therapies include:
Outcomes-based agreements tied to biomarker response
Milestone payments based on functional improvements
Annuity models spreading costs over multi-year periods
Clinical Trial Landscape: ClinicalTrials.gov Analysis
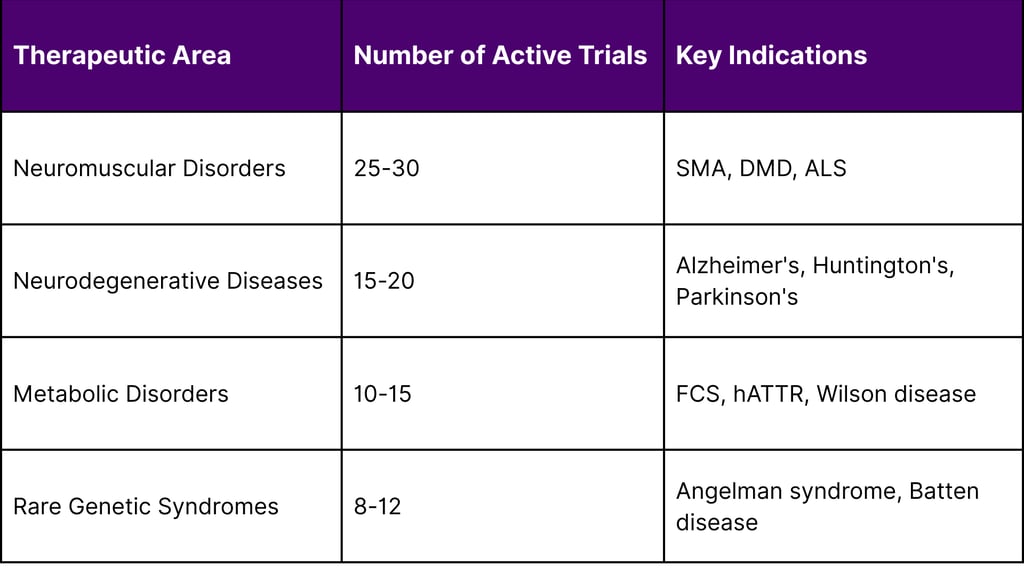
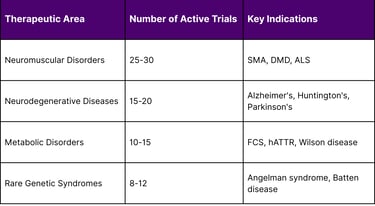
As of December 2025, over 70 pharmaceutical companies are actively developing antisense oligonucleotide therapeutics across multiple therapeutic areas:
Active Phase 2/3 Clinical Trials by Therapeutic Area
Challenges and Future Directions
Remaining Scientific Hurdles
Despite remarkable progress, several challenges persist:
Delivery Optimization: While BBB penetration has improved, consistent delivery to deep brain structures and peripheral nerve tissues remains challenging.
Duration of Action: Although current ASOs demonstrate months-long activity, developing ultra-long-acting formulations could reduce treatment burden further.
Off-Target Effects: Continued refinement of chemical modifications to minimize unintended gene interactions while maintaining therapeutic potency.
Scalable Manufacturing: Developing cost-effective manufacturing processes for individualized ASOs remains complex.
Integration with Other Modalities
The future of rare disease treatment likely involves combination approaches:
ASOs with gene therapy for complementary mechanisms
ASOs with small molecules to address multiple pathogenic pathways
ASOs with enzyme replacement therapy for metabolic disorders
Recommendations for Stakeholders
For R&D Organizations
Invest in platform technologies that enable rapid target switching
Prioritize blood-brain barrier penetration research
Develop robust biomarker strategies for accelerated approval pathways
Engage with FDA early through pre-IND meetings
For Clinical Development Teams
Design adaptive trial protocols accommodating small patient populations
Implement basket trial designs for similar mechanistic approaches across indications
Leverage natural history studies and patient registries
Establish relationships with patient advocacy groups early
For Market Access Professionals
Develop comprehensive health economic models demonstrating lifetime value
Prepare for outcomes-based contracting negotiations
Engage payers early in development process
Document caregiver burden and quality of life impacts
Conclusion
ASO 2.0 represents a fundamental shift in how medicine approaches rare genetic disorders. The convergence of advanced chemical platforms, innovative delivery technologies, supportive regulatory frameworks, and sustainable reimbursement models is creating an unprecedented opportunity to address diseases once considered untreatable.
As blood-brain barrier penetration becomes routine, pediatric approvals accelerate, and individualized ASO development streamlines, the field is poised for exponential growth through 2026 and beyond. The next generation of antisense therapies will not merely treat rare diseases they will redefine what is possible in precision medicine.
For stakeholders across R&D, clinical development, and market access, the message is clear: ASO technology has matured from experimental to essential. Organizations that invest strategically in this platform today will be positioned to transform patient lives tomorrow.
Frequently Asked Questions (FAQs)
Q1: What makes ASO 2.0 different from first-generation antisense oligonucleotides?
ASO 2.0 incorporates advanced chemical modifications (2'-MOE, PS backbones, PMO), improved delivery systems (GalNAc conjugation, BBB-penetrating peptides), longer duration of action (3-6 month dosing intervals), and better safety profiles. These improvements address the nuclease degradation, poor cellular uptake, and frequent dosing requirements that limited first-generation ASOs.
Q2: How do ASOs cross the blood-brain barrier?
Several strategies enable BBB penetration: ApoE-derived peptide conjugates that utilize natural transport mechanisms, transferrin receptor targeting through oligonucleotide transfer vectors, and glucose transporter-mediated delivery using glucose-coated nanocarriers. These approaches can achieve 78% brain parenchyma penetration following intravenous administration, potentially eliminating the need for invasive intrathecal injections.
Q3: What is the FDA's Platform Technology Designation and why does it matter for ASO development?
Introduced in 2023, Platform Technology Designation provides expedited development pathways for well-characterized ASO platforms. It recognizes that oligonucleotide safety profiles depend primarily on chemical structure rather than target sequence, allowing custom therapies to reference platform toxicology studies. This dramatically reduces development timelines for new ASO applications.
Q4: How many ASO therapies are currently FDA-approved?
As of early 2026, the FDA has approved 17 RNA oligonucleotide therapeutics, including 11 antisense oligonucleotides and 6 siRNAs. These therapies primarily target rare genetic disorders, including neuromuscular diseases (SMA, DMD), neurodegenerative conditions (SOD1-ALS, hATTR amyloidosis), and metabolic disorders (FCS).
Q5: What is "n-of-1" medicine and how do ASOs enable it?
N-of-1 medicine refers to therapies designed for individual patients or very small patient populations with unique genetic variants. ASOs enable this through their sequence-specific mechanism and the FDA's individualized ASO guidance framework, which streamlines development for severely debilitating or life-threatening diseases affecting typically one or two prospectively identified individuals.
Q6: What role do biomarkers play in ASO clinical development?
Biomarkers are critical for ASO development, particularly for rare diseases where traditional clinical endpoints are challenging. The FDA's accelerated approval pathway accepts biomarkers like CSF neurofilament light chains (for neurodegenerative diseases) and target protein levels as evidence of clinical benefit, enabling faster approvals with confirmatory trials conducted post-approval.
Q7: How long do ASO treatments last, and how often are they administered?
Modern ASOs demonstrate extended duration of action due to their stability in tissues. Current approved ASOs are administered every 1-6 months depending on the specific product and indication. Next-generation splice-switching ASOs under development may enable dosing every 9-12 months, significantly reducing treatment burden.
Q8: What is the cost outlook for ASO therapies?
ASO pricing reflects small patient populations, high R&D costs, and complex manufacturing. However, emerging value-based contracting models, outcomes-based agreements, and annuity payment structures are making these therapies more accessible. The projected incremental market growth of $10.7 billion from 2023-2033 suggests sustainable pricing models are emerging.
Q9: Which rare diseases are most likely to benefit from ASO therapies?
ASOs are particularly suited for: single-gene disorders with known genetic mutations, diseases where reducing toxic protein is beneficial (Huntington's disease, ALS), conditions requiring increased functional protein (SMA), and disorders amenable to exon skipping (DMD). Current pipelines focus heavily on neuromuscular, neurodegenerative, and metabolic rare diseases.
Q10: What is the outlook for ASO therapies in 2026 and beyond?
The outlook is highly promising. Expected 2026 developments include: pivotal Phase 3 data for Angelman syndrome and other pediatric conditions, multiple regulatory submissions for new indications, continued advancement of BBB-penetrating delivery systems, and expansion beyond rare diseases into chronic conditions like cardiovascular disease and cancer. The convergence of technology, regulatory support, and clinical success positions ASOs for significant growth.
References
American Society for Clinical Investigation. (2024). The expanding application of antisense oligonucleotides to neurodegenerative diseases. Journal of Clinical Investigation.
Cummings, J. (2025). Alzheimer's disease drug development pipeline: 2025. Alzheimer's & Dementia: Translational Research & Clinical Interventions.
National Institutes of Health. (2025). Genetic and Rare Diseases Information Center.
National Organization for Rare Disorders. (2025). America's Rare Children Need Congress to Act: NORD Urges Swift Reauthorization of Proven Rare Pediatric Disease Voucher Program.
Sang, A., Zhuo, S., Bochanis, A., McGowan, J. E., Bardhan, R., Zhou, X., & Rader, T. P. (2024). Mechanisms of action of the US Food and Drug Administration-approved antisense oligonucleotide drugs. BioDrugs, 38(4), 511-526. doi: 10.1007/s40259-024-00665-2
Ultragenyx Pharmaceutical Inc. (2026). Ultragenyx Provides Financial and Business Updates at J.P. Morgan Annual Healthcare Conference.
U.S. Food and Drug Administration. (2025). 2025 FDA approvals. Nature Reviews Drug Discovery.
U.S. Food and Drug Administration. (2025). CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 219407Orig1s000.
U.S. Food and Drug Administration. (2025). Guidance Documents for Rare Disease Drug Development.
U.S. Food and Drug Administration. (2025). Nonclinical Safety Assessment of Oligonucleotide-Based Therapeutics.
Wakap, S. N., Lambert, D. M., Olry, A., Rodwell, C., Gueydan, C., Lanneau, V., Murphy, D., Cam, Y. L., & Rath, A. (2020). Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. European Journal of Human Genetics, 28(2), 165-173. doi: 10.1038/s41431-019-0508-0
Zamecnik, P. C., & Stephenson, M. L. (1978). Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proceedings of the National Academy of Sciences, 75(1), 280-284.

