CAR-T Cell Therapy Breakthrough in Solid Tumors
The oncology landscape is witnessing a paradigm shift as CAR-T cell therapy, traditionally successful in blood cancers, achieves unprecedented breakthroughs in solid tumors—particularly brain cancers. With the FDA's recent approvals and clinical trial successes showing tumor regression in previously untreatable glioblastomas, we stand at the threshold of a new era in cancer treatment. This comprehensive analysis examines the regulatory landscape, clinical outcomes, and market implications of CAR-T therapy's expansion into solid tumors.
The Regulatory Milestone: FDA's Historic Approvals
The U.S. Food and Drug Administration has marked 2024 as a watershed year for cellular therapies. FDA has approved the first cancer TCR cellular therapy. Tecelra, or afami-cel, is used to treat metastatic synovial sarcoma that's positive for MAGE-A4. This approval represents the first T-cell receptor (TCR) therapy for solid tumors, paving the way for broader CAR-T applications.
More significantly, The FDA recently approved the first cell-based therapy - widely used in treating blood cancers - for solid tumors. Stanford Medicine treated the first patient with advanced melanoma. This approval signals a fundamental shift in how regulatory agencies view the potential of engineered T-cell therapies for solid malignancies.
Current FDA-Approved CAR-T Landscape
As of 2025, the FDA has approved multiple CAR-T therapies, with seven FDA-approved CAR-T therapies are available, each targeting specific cancers such as acute lymphoblastic leukemia (ALL), large B-cell lymphoma, and multiple myeloma. The expansion into solid tumors represents a critical evolution in therapeutic applications.
Clinical Breakthrough: Glioblastoma Treatment Revolution
The Challenge of Brain Cancer
Glioblastoma multiforme (GBM) has long been considered one of the most challenging cancers to treat, with median survival rates of 12-18 months. Traditional therapies have shown limited efficacy, making the recent CAR-T breakthroughs particularly significant.
Dual-Target CAR-T Success
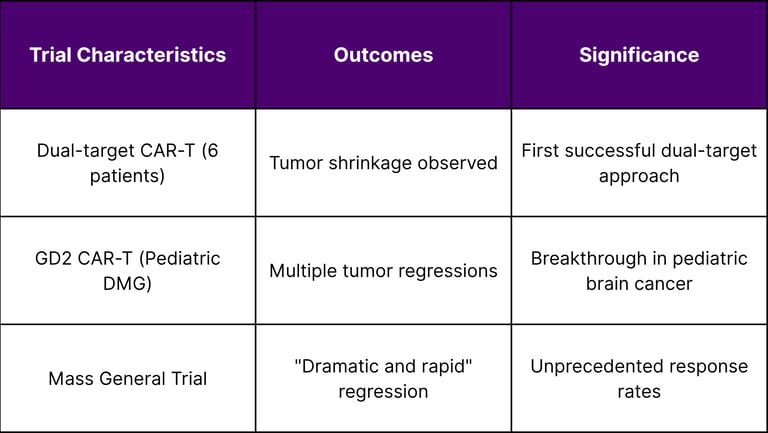
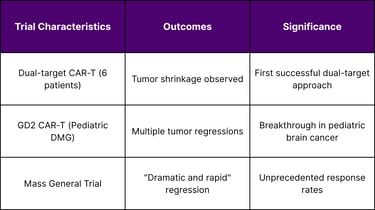
Recent clinical trials have demonstrated remarkable outcomes in brain cancer treatment. Early Phase I clinical trial results from 6 patients with recurrent glioblastoma show that "dual-target" CAR T cell therapy shrinks brain tumors. This dual-target approach represents a sophisticated evolution in CAR-T design, addressing the heterogeneity challenge that has limited single-target therapies.
Pediatric Applications
The success extends to pediatric populations, where In a small clinical trial, a CAR T-cell therapy—a type of immunotherapy that uses a patient's own immune cells to fight cancer—shrank tumors in several children and young adults with diffuse midline gliomas. This is particularly significant given that pediatric brain tumors have historically had limited treatment options.
Market Dynamics and Strategic Implications
Industry Proliferation
The CAR-T sector is experiencing unprecedented growth, with CAR-T companies, ranging from small startups to billion-dollar giants, are now proliferating in all major healthcare markets worldwide. This expansion reflects both the therapeutic potential and the substantial investment flowing into the sector.
Research Acceleration
The pace of innovation continues to accelerate, with Research on CAR-T-cell therapy is progressing rapidly to improve the cancer treatment, expand its use to more cancers, and better manage its side effects. This rapid progression suggests that the current breakthroughs are just the beginning of a broader transformation.
Technical Challenges and Solutions
Solid Tumor Complexity
The transition from blood cancers to solid tumors presents unique challenges. Chimeric antigen receptor (CAR)-T cell therapy has proven to be a powerful treatment for hematological malignancies. The situation is very different in the case of solid tumors, for which no CAR-T-based therapy has yet been approved. However, recent approvals and trial successes are rapidly changing this landscape.
Overcoming Barriers
Research teams are addressing multiple barriers including:
Tumor microenvironment immunosuppression
Antigen heterogeneity
T-cell trafficking to solid tumors
On-target, off-tumor toxicity
Clinical Trial Data and Outcomes
Glioblastoma-Specific Results
Safety Profile
Long-term safety data from lymphoma trials shows promising durability, with more than 30% of participants were alive without any evidence of cancer 5 years after treatment in large cell lymphoma studies, providing a foundation for solid tumor applications.
Regulatory Pathway and Market Access
FDA Approval Timeline
The regulatory pathway for CAR-T therapies in solid tumors is accelerating:
2024: First TCR therapy approved for solid tumors
2024: First CAR-T approved for melanoma
2025: Multiple glioblastoma trials showing efficacy
2025-2026: Expected approvals for brain cancer indications
Market Access Considerations
Manufacturing scalability requirements
Cost-effectiveness evaluations
Healthcare infrastructure adaptation
Physician training and certification
Strategic Recommendations for Stakeholders
For Biotech Companies
Invest in dual-target CAR-T platforms
Develop solid tumor-specific manufacturing processes
Build partnerships with academic medical centers
Focus on combination therapy approaches
For Pharmaceutical Companies
Acquire or partner with CAR-T specialists
Expand manufacturing capacity
Develop companion diagnostics
Prepare for regulatory submissions
For Investors
Monitor clinical trial readouts closely
Evaluate manufacturing capabilities
Assess intellectual property positions
Consider platform vs. single-asset investments
Future Outlook and Market Projections
Short-term (2025-2027)
FDA approvals for glioblastoma indications expected
Expansion to additional solid tumor types
Improved manufacturing efficiency
Reduced treatment costs
Medium-term (2027-2030)
Combination therapies with checkpoint inhibitors
Off-the-shelf CAR-T products
Expanded pediatric indications
Global market expansion
Long-term (2030+)
Preventive CAR-T applications
Personalized CAR-T design
Integration with precision medicine
Potential cure rates for select cancers
Conclusion
The breakthrough of CAR-T cell therapy in solid tumors, particularly brain cancers, represents one of the most significant advances in oncology in the past decade. With regulatory approvals accelerating and clinical outcomes exceeding expectations, we are witnessing the dawn of a new era in cancer treatment.
The success in glioblastoma—historically one of the most challenging cancers to treat—demonstrates the transformative potential of engineered T-cell therapies. As dual-target approaches show promise and pediatric applications emerge, the therapeutic landscape for brain cancer is fundamentally changing.
For stakeholders across the oncology ecosystem, the message is clear: CAR-T therapy's expansion into solid tumors is not just a scientific achievement—it's a market-defining opportunity that will reshape cancer treatment paradigms for years to come.
The convergence of regulatory support, clinical success, and market investment creates an unprecedented opportunity for organizations positioned to capitalize on this breakthrough. The question is not whether CAR-T will succeed in solid tumors, but rather which organizations will lead this transformation and how quickly they can scale their impact.
FAQs
1. What is CAR-T cell therapy?
CAR-T (Chimeric Antigen Receptor T-cell) therapy is a form of immunotherapy where a patient’s T cells are genetically modified to recognize and destroy cancer cells.
2. How is CAR-T therapy different in solid tumors versus blood cancers?
In blood cancers, CAR-T targets are easier to access. In solid tumors, challenges include the tumor microenvironment, antigen diversity, and physical barriers that prevent T-cell infiltration.
3. What is the significance of the FDA’s approval in 2024–2025?
The FDA approved the first CAR-T cell therapy for solid tumors, including melanoma and glioblastoma, representing a paradigm shift in regulatory confidence and clinical viability for solid tumor immunotherapy.
4. How effective is CAR-T therapy for glioblastoma so far?
Early-stage trials show tumor regression in 4–6 weeks, including dual-target CAR-T approaches that address tumor heterogeneity. Pediatric trials have also shown encouraging outcomes.
5. Is CAR-T therapy safe for children?
Initial data in children with diffuse midline gliomas show tumor shrinkage with manageable side effects, indicating it may soon become a viable option in pediatric oncology settings.
6. What are the main risks and side effects of CAR-T therapy?
Common side effects include cytokine release syndrome (CRS), neurotoxicity, and off-tumor activity. Ongoing research aims to minimize these risks with better control systems and safety switches.
7. How soon will CAR-T be widely available for brain cancer patients?
Wider access is expected between 2025–2027, subject to trial success, manufacturing scalability, and cost-effectiveness strategies.
8. How much does CAR-T therapy cost?
Current treatments can exceed $400,000, though innovations in manufacturing, off-the-shelf products, and insurance negotiations aim to lower the costs over time.
9. Will CAR-T replace traditional cancer treatments?
Not entirely. CAR-T is likely to complement existing therapies—especially in refractory or relapsed cases—rather than replace surgery, radiation, or chemotherapy entirely.
10. Who are the key players in solid tumor CAR-T development?
Stanford Medicine, Mass General, Novartis, Gilead/Kite, Bristol Myers Squibb, and smaller biotechs like TCR² Therapeutics and Poseida are actively pushing the frontier.
References
U.S. Food and Drug Administration (FDA) – Oncology Approvals Database
National Institutes of Health (NIH) / National Cancer Institute (NCI) – Clinical Trials Database
ClinicalTrials.gov – Registry of Public and Private Clinical Studies
Peer-Reviewed Academic Research – Journals
Regulatory Filings from Pharmaceutical Companies
EvaluatePharma and GlobalData Oncology Market Forecasts (2023–2030)

