Clinical Trial Decentralization


The landscape of rare disease clinical trials is undergoing a fundamental transformation. With more than 10,000 known rare diseases affecting approximately 30 million people in the United States alone, and only about 500 having approved treatments, the urgency for innovative therapeutic development has never been greater. Decentralized Clinical Trials (DCTs) have emerged as a strategic imperative, offering unprecedented opportunities to accelerate drug development, enhance patient engagement, and overcome longstanding barriers in rare disease research.
The Rare Disease Challenge: A Public Health Crisis
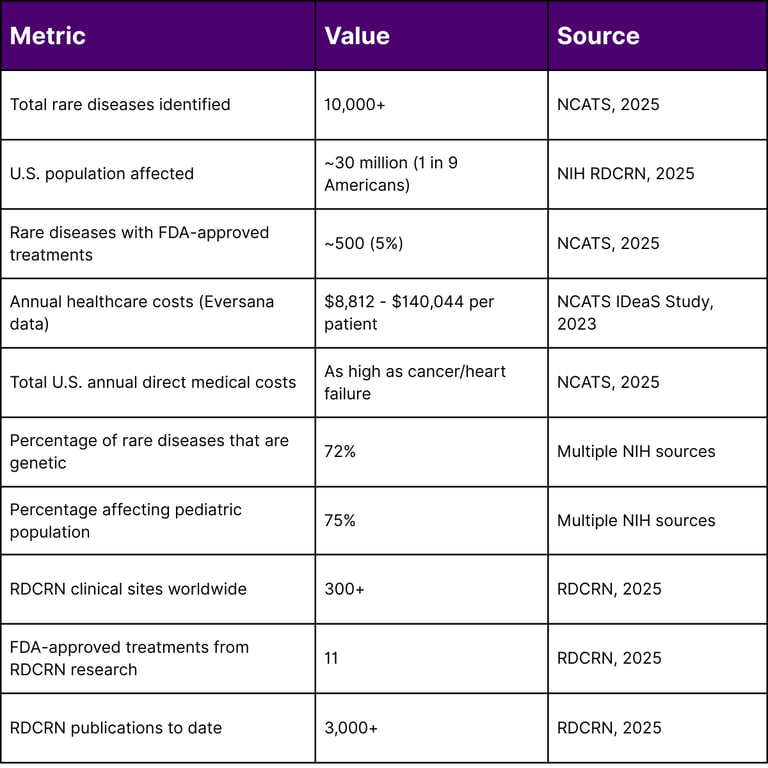
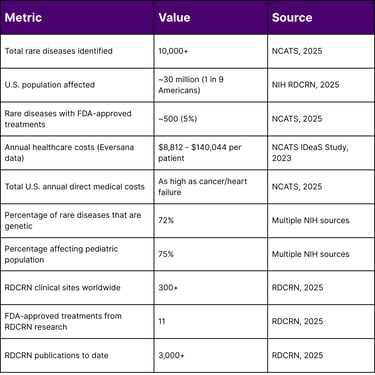
Rare diseases represent a significant public health burden that extends far beyond their individual prevalence rates. According to the National Center for Advancing Translational Sciences (NCATS), rare diseases collectively affect nearly one in every nine Americans, with most conditions starting in childhood. The economic impact is staggering, with research suggesting that nationwide medical costs for individuals with rare diseases are likely as high as those faced by people with common diseases such as cancer and heart failure.
Key Statistics on Rare Disease Burden
The challenges in rare disease clinical trials are well-documented. Research published through the National Institutes of Health indicates that approximately 30% of Phase 3 studies fail due to enrollment challenges. The inherently small and geographically dispersed patient populations, combined with stringent eligibility criteria, create significant recruitment and retention obstacles that traditional site-based trial models struggle to overcome.
The Decentralized Clinical Trial Revolution
Regulatory Foundation and FDA Guidance
In September 2024, the U.S. Food and Drug Administration issued final guidance on "Conducting Clinical Trials with Decentralized Elements," providing a comprehensive regulatory framework that validates and encourages the adoption of decentralized approaches. This guidance represents a watershed moment for the clinical research industry, particularly for rare disease development.
The FDA defines decentralized clinical trials as studies that include decentralized elements where trial-related activities occur at locations other than traditional clinical trial sites. These elements may include:
Telehealth visits with trial personnel
In-home visits with remote trial personnel or local healthcare providers
Digital health technologies (DHTs) for remote data collection and monitoring
Local laboratory facilities for specimen collection and analysis
Direct-to-patient shipment of investigational products
The guidance emphasizes two fundamental principles that must underpin all decentralized elements: patient safety and data integrity. Importantly, the FDA explicitly supports both fully decentralized trials (where all activities occur remotely) and hybrid models that combine traditional site visits with decentralized elements.
The Growth and Evolution of DCTs
The adoption of decentralized approaches has demonstrated sustained momentum since the first DCT was conducted in 2011. Analysis of the ClinicalTrials.gov database reveals that since the outbreak of COVID-19 in 2020, more than 500 DCTs have been conducted annually. The pandemic catalyzed this growth, but the sustained adoption reflects recognition of DCTs' intrinsic value, particularly for rare disease populations.
Research published in 2025 by the American Statistical Association Biopharmaceutical Section examined DCT implementation through March 2024, identifying diverse applications across therapeutic areas including medication, vaccine, and digital interventions. The analysis demonstrates that DCTs are particularly suitable for trials involving chronic diseases, rare diseases, immobile participants, self-administered investigational products, and lower-risk-profile products.
Strategic Advantages for Rare Disease Development
1. Enhanced Patient Recruitment and Retention
One of the most compelling advantages of DCTs in rare disease research is their ability to dramatically expand the accessible patient pool. Traditional clinical trials require patients to travel sometimes across state lines or international borders to specialized centers, creating insurmountable barriers for many potential participants.
According to research funded by the NIH Rare Diseases Clinical Research Network (RDCRN), patient recruitment remains the primary challenge in rare disease trials. Studies show that over 25% of rare disease clinical trials terminate due to low patient accrual rates, and approximately 30% of Phase 3 studies fail due to enrollment challenges. DCTs address this challenge by:
Eliminating geographic barriers: Patients can participate regardless of proximity to specialized centers
Reducing travel burden: Particularly crucial for pediatric populations (75% of rare diseases) where families must coordinate school schedules and work obligations
Increasing accessibility: Patients with mobility limitations or severe illness who cannot safely travel can participate remotely
Improving retention: The convenience of at-home or local participation significantly reduces dropout rates
2. Accelerated Timeline to Market
The small sample sizes inherent to rare disease trials, while reducing statistical power, can be partially offset through enhanced recruitment efficiency. Analysis of rare disease trials registered on ClinicalTrials.gov reveals that phase 2 trials for the rarest diseases (prevalence <1/1,000,000) averaged only 15.7 participants, while phase 3 trials averaged 19.2 participants.
DCTs can compress recruitment timelines by:
Enabling simultaneous enrollment across multiple geographies without requiring physical site infrastructure
Reducing site activation times through streamlined remote protocols
Facilitating rapid participant identification through digital outreach and patient registries
Minimizing scheduling conflicts that often delay enrollment in traditional trials
3. Improved Data Quality and Diversity
Contrary to initial concerns about data variability, DCTs can actually enhance data quality through:
Real-world data capture: Measurements taken in patients' natural environments may better reflect true disease burden
Continuous monitoring: Wearable devices and digital health technologies enable more frequent data collection than periodic site visits
Reduced missing data: The convenience of remote participation improves protocol adherence
Enhanced diversity: By removing geographic and socioeconomic barriers, DCTs can recruit more representative study populations
Analysis of clinical trials across various rare diseases found that digital health technologies enable remote monitoring, digital interventions, and patient support, with notable increases in DHT adoption from 2017 through 2024 across nearly all disease areas.
4. Cost Optimization
While DCTs require investment in technology infrastructure and remote monitoring systems, they offer significant cost savings through:
Reduced site infrastructure costs
Lower patient reimbursement expenses (reduced travel costs)
Decreased protocol deviations and associated remediation costs
Improved retention reducing costs associated with replacing dropouts
More efficient use of clinical research staff time
The "4A" Framework for DCT Implementation
Based on systematic analysis of rare disease trials utilizing digital health technologies, a comprehensive framework for successful DCT implementation has emerged, centered on four key principles:
Accessibility
Expanding trial participation beyond traditional geographic and socioeconomic constraints. This includes providing sponsor-supplied devices for participants who lack necessary technology, ensuring multilingual support, and designing protocols that accommodate varying levels of digital literacy.
Agility
Building flexibility into trial design to adapt to emerging data, patient feedback, and operational challenges. This includes adaptive randomization strategies, interim analyses, and protocol amendments that can respond to real-world implementation experiences.
Awareness
Leveraging patient advocacy organizations, digital communities, and social media to raise awareness about trial opportunities and facilitate recruitment. Research published through NIH demonstrates that direct-to-consumer recruitment methods via traditional and social media can significantly aid research accrual for rare disease clinical trials.
Adaptability
Customizing decentralized elements to specific disease characteristics, patient populations, and therapeutic interventions. Not all trials benefit equally from decentralization decisions should be driven by careful assessment of patient needs, safety requirements, and data collection necessities.
Implementation Considerations and Best Practices
Protocol Design
The FDA guidance emphasizes that sponsors should consider decentralized elements during initial protocol development rather than as afterthoughts. Key considerations include:
Visit Type Appropriateness: Determining whether each study visit can be conducted via telehealth, requires in-person assessment by local providers, or necessitates attendance at a specialized site.
Investigational Product Characteristics: Products with well-characterized safety profiles and simple administration may be suitable for home-based delivery and administration, while those requiring complex preparation or close medical supervision may necessitate site visits. The FDA's final guidance specifically notes that fully decentralized trials may be appropriate for investigational products with well-characterized safety profiles that do not require complex preparation, administration, or medical assessment.
Patient Population: Considerations include age (pediatric vs. adult), disease severity, cognitive capacity, and ability to safely participate in remote procedures.
Technology Infrastructure
Successful DCT implementation requires robust technology platforms for:
Telehealth capabilities: HIPAA-compliant video conferencing for remote visits
Electronic data capture: Real-time data collection with built-in quality checks
Digital health technologies: FDA guidance explicitly supports "Bring Your Own Device" (BYOD) approaches while requiring sponsors to provide devices to participants who lack them
Remote monitoring: Platforms for continuous oversight of study conduct and participant safety
Roles and Responsibilities
The FDA guidance clarifies that sponsors retain ultimate responsibility for trial conduct regardless of decentralization. Specific responsibilities include:
Sponsors: Must ensure proper coordination of all decentralized elements, implement risk-based monitoring plans, develop comprehensive data management plans tracking all data sources, and maintain records of all contracted service providers.
Investigators: Remain responsible for reviewing data from local healthcare providers and remote personnel, ensuring quality and consistency, and maintaining a physical location where records are accessible for FDA inspection.
Local Healthcare Providers (HCPs): May conduct trial-related activities that are part of routine clinical practice without being designated as sub-investigators, provided they maintain appropriate documentation. The final guidance emphasizes that trial-related activities performed by local HCPs should be designed to limit variability in data collected by including specific instructions in the protocol.
Overcoming Barriers to Implementation
Regulatory Complexity
While the FDA's 2024 guidance provides clarity, navigating state-level telehealth regulations and international requirements remains challenging. Careful consideration of state telehealth laws and regulations remains critical when conducting DCTs via telehealth. Ensuring uniform application of protocols across state lines to generate consistent data despite variations in state telehealth laws and practice standards is a key component of conducting clinical trials with decentralized elements.
Best practices include:
Early engagement with regulatory authorities
Careful documentation of state-specific compliance strategies
Consultation with legal experts on cross-border data transfer requirements
Proactive communication with Institutional Review Boards (IRBs)
Data Security and Privacy
Protecting participant data in decentralized settings requires:
End-to-end encryption for all remote data transmission
Clear policies on data storage and retention
Participant education on privacy protection measures
Compliance with 21 CFR Part 11 for electronic records (the final guidance clarifies that while audio/video telehealth interactions do not need to be 21 CFR Part 11 compliant, all study data captured during virtual visits must conform to these requirements)
Robust audit trails capturing all data access
Stakeholder Education
Successful DCT implementation requires buy-in and competency across all stakeholder groups:
Patients and caregivers: Training on technology use and protocol requirements
Site staff: Education on remote monitoring procedures and responsibilities
Local healthcare providers: Clear guidance on their specific roles and documentation requirements
Sponsors and CROs: Building institutional expertise in DCT management
Case Studies: DCTs in Rare Disease Research
Example 1: Cystic Fibrosis Remote Monitoring Study
A Phase 4 study investigated wearable technology capabilities for monitoring physical activity, cough frequency, and sleep quality in cystic fibrosis patients taking Elexacaftor/Tezacaftor/Ivacaftor. All visits were conducted via telehealth using a mobile app, demonstrating the feasibility of fully remote data collection for chronic rare disease management.
Example 2: Target Rare Cancer Knowledge (TRACK) Trial
ClinicalTrials.gov identifier NCT04504604 represents an innovative fully remote and decentralized study leveraging personalized genomic strategies. Driven by patient advocates, this trial eliminates facility visits for enrollment or treatment, aiming to enroll 400 participants using remote consent processes and guidance from a Molecular Tumor Board.
Example 3: NIH Rare Diseases Clinical Research Network
The RDCRN, established under the Rare Diseases Act of 2002, coordinates research on nearly 200 rare diseases through 21 distinct consortia. In fiscal year 2025, NIH awarded approximately $26 million in grants for RDCRN's fifth funding cycle, emphasizing approaches that expand trial accessibility. The network now consists of 21 research consortia, including 10 joining for the first time, five renewed from the previous cycle, and six continuing through one-year extensions.
Since its establishment, the RDCRN has supported hundreds of studies at over 300 clinical sites worldwide, resulting in over 3,000 publications and contributing to the FDA approval of 11 treatments for rare diseases.
Future Directions and Emerging Trends
Integration of Artificial Intelligence
Machine learning algorithms are increasingly being deployed to:
Identify eligible patients through electronic health record analysis
Predict protocol deviations before they occur
Optimize trial designs through simulation
Analyze complex multi-modal data from digital health technologies
Regulatory Harmonization
The European Medicines Agency published recommendations on decentralized elements in December 2022, and international harmonization efforts continue. A 2024 analysis comparing FDA and EMA guidance found that both regulatory bodies commonly emphasized assessment of the appropriateness of decentralized elements along two axes: patient safety and data integrity. Convergence between FDA and EMA guidance will facilitate global rare disease trials and accelerate access to therapies.
Platform Trials and Master Protocols
For rare diseases, platform trials that evaluate multiple therapies within a single infrastructure offer unprecedented efficiency. Combined with decentralized elements, these designs can dramatically reduce the time and cost of therapeutic development.
The Competitive Imperative
For pharmaceutical and biotechnology companies, embracing clinical trial decentralization is no longer optional it represents a strategic competitive advantage in rare disease development. Organizations that successfully implement DCTs will:
Accelerate time to market: Faster recruitment and data collection compress development timelines
Reduce development costs: Operational efficiencies translate to lower per-patient costs
Improve regulatory success: Higher quality, more diverse data strengthens submissions
Build patient community relationships: Patient-centric approaches foster loyalty and advocacy
Attract investment: Demonstrated DCT competency signals operational excellence
Conclusion
Clinical trial decentralization represents a fundamental shift in how rare disease research is conducted. The convergence of regulatory support, technological capability, patient demand, and economic incentives has created a unique moment of opportunity. The FDA's 2024 guidance provides a clear roadmap, removing uncertainty that previously inhibited widespread adoption.
For the approximately 30 million Americans living with rare diseases and the hundreds of millions affected globally DCTs offer hope for accelerated therapeutic development. For sponsors, they provide a path to operational excellence and competitive differentiation in an increasingly crowded rare disease market.
The question is no longer whether to implement decentralized approaches, but how quickly and effectively organizations can build the capabilities necessary to execute them at scale. Those who move decisively will define the future of rare disease drug development.
Frequently Asked Questions (FAQ)
Q: What is the difference between a fully decentralized and a hybrid clinical trial?
A: A fully decentralized clinical trial conducts all trial-related activities remotely, with no requirement for participants to visit traditional clinical trial sites. A hybrid trial combines traditional on-site visits with decentralized elements. According to the FDA guidance, the choice depends on the investigational product characteristics, patient population needs, and specific trial activities required.
Q: Are decentralized clinical trials accepted by regulatory agencies?
A: Yes. The FDA's September 2024 final guidance explicitly supports both fully decentralized and hybrid trials, provided they maintain patient safety and data integrity. The European Medicines Agency issued similar recommendations in December 2022. Both agencies emphasize that DCTs should be assessed on the same standards as traditional trials.
Q: How do DCTs address patient diversity and health equity?
A: DCTs reduce geographic, logistic, and economic barriers that disproportionately affect underrepresented populations. By eliminating travel requirements and enabling participation from home or local facilities, DCTs can recruit more diverse study populations that better represent the true patient community. The FDA specifically highlights DCTs' potential to improve clinical trial accessibility and diversity.
Q: What are the main challenges in implementing DCTs for rare diseases?
A: Key challenges include: navigating varying state and international regulations for telehealth; ensuring all participants have access to necessary technology; training local healthcare providers on protocol-specific procedures; maintaining data security across multiple remote locations; and managing the complexity of coordinating decentralized activities. However, these challenges are increasingly addressed through improved technology platforms and operational experience.
Q: How is data quality maintained in decentralized trials?
A: The FDA guidance requires sponsors to implement risk-based monitoring plans specifically addressing decentralized elements, comprehensive data management plans tracking all data sources, and safety monitoring plans accounting for remote participation. Digital health technologies often enable more frequent and continuous data collection than traditional site visits, potentially improving data completeness and quality.
Q: Can investigational products be shipped directly to patients?
A: Yes, provided the product's safety profile and handling requirements allow it. The FDA guidance states that investigational products can be dispensed remotely or by local healthcare providers if carefully evaluated according to the product's safety characteristics. This requires robust tracking systems and clear protocols for storage, handling, and accountability.
Q: What role do patient advocacy organizations play in DCTs?
A: Patient advocacy organizations are crucial partners in DCT success. They help with trial design by providing patient perspective, facilitate recruitment through their networks, raise awareness about trial opportunities, and provide ongoing support to participants. The RDCRN works closely with patient advocacy groups to study nearly 200 rare diseases, demonstrating the value of these partnerships.
Q: How do DCTs impact trial timelines and costs?
A: While DCTs require upfront investment in technology infrastructure, they typically compress overall timelines through faster recruitment, reduced scheduling conflicts, and improved retention. Enhanced efficiency and reduced site infrastructure costs can lower overall development expenses, though specific impacts vary by study design and disease characteristics.
Q: Are there certain types of rare disease trials that are not suitable for decentralization?
A: Yes. Trials requiring complex investigational product administration (such as cell or gene therapy), specialized imaging (MRI, CT scans), or detailed physical examinations by subspecialists may require traditional site visits. However, even these trials can benefit from hybrid approaches where follow-up visits and data collection occur remotely while key procedures are conducted at sites.
Q: How can sponsors ensure compliance with 21 CFR Part 11 in DCTs?
A: The FDA guidance clarifies that while audio/video telehealth interactions do not need to be 21 CFR Part 11 compliant, all study data captured during virtual visits must conform to these electronic records requirements. This requires validated electronic data capture systems with appropriate controls, secure authentication, comprehensive audit trails, and electronic signatures where needed.
References
Augustine, E. F., Adams, H. R., & Mink, J. W. (2013). Clinical trials in rare disease: Challenges and opportunities. Journal of Child Neurology, 28(9), 1142–1150.
Bell, S. A., & Tudur Smith, C. (2014). A comparison of interventional clinical trials in rare versus non-rare diseases: An analysis of ClinicalTrials.gov. Orphanet Journal of Rare Diseases, 9, 170.
Bloem, L. T., Ogungbenro, K., Al-Salami, H., Aarons, L., & Gad, S. C. (2018). Characteristics of clinical trials in rare vs. common diseases: A register-based Latvian study. PLOS ONE, 13(4).
Cassiman, D., Cornet, L., Kumps, C., De Waele, L., & Wuyts, W. (2024). iSTORE: A project on innovative statistical methodologies to improve rare diseases clinical trials in limited populations. Orphanet Journal of Rare Diseases, 19.
Gagne, J. J., Thompson, L., O'Keefe, K., & Kesselheim, A. S. (2014). Innovative research methods for studying treatments for rare diseases: Methodological review. BMJ, 349.
Genetic and Rare Diseases Information Center. (2025). Home. National Institutes of Health.
Hee, S. W., Willis, A., Tudur Smith, C., Day, S., Miller, F., Madan, J., et al. (2017). Recommendations for the design of small population clinical trials. Orphanet Journal of Rare Diseases, 12, 195.
National Center for Advancing Translational Sciences. (2023). Illuminating the druggable genome: Insights into drug development for rare diseases – Fact sheet. National Institutes of Health.
National Center for Advancing Translational Sciences. (2025). Our impact on rare diseases. National Institutes of Health.
National Center for Advancing Translational Sciences. (2025). Rare Disease Day at NIH. National Institutes of Health.
National Institutes of Health. (2024). Transformation of the clinical trial enterprise: Lessons learned from the COVID-19 pandemic—Decentralized clinical trials and digital health technologies. NCBI Bookshelf.
National Institutes of Health. (2025). Rare diseases.
Park, J., Yu, K. S., Huh, K. Y., & Chung, W. K. (2024). The landscape of decentralized clinical trials (DCTs): Focusing on the FDA and EMA guidance. Translational and Clinical Pharmacology, 32(1).
Quattrocchi, A., Banus, C., Grieve, A., & Breckenridge, A. M. (2023). Decentralized clinical trials and rare diseases: A Drug Information Association Innovative Design Scientific Working Group (DIA-IDSWG) perspective. Orphanet Journal of Rare Diseases, 18, 86.
Rare Diseases Clinical Research Network. (2025). Home. National Institutes of Health.
Rare Diseases Clinical Research Network. (2025). NIH announces funding to establish and strengthen rare disease research groups. National Institutes of Health.
Song, Y., Xu, Z., Zhou, J., Lu, N., Cheng, Q., & Zhao, Y. (2022). Novel clinical trial design and analytic methods to tackle challenges in therapeutic development in rare diseases. Annals of Translational Medicine, 10(20).
U.S. Food and Drug Administration. (2024). Conducting clinical trials with decentralized elements: Guidance for industry, investigators, and other interested parties.
Wang, S., et al. (2025). Decentralized clinical trials in the era of real-world evidence: A critical assessment of recent experiences. Clinical and Translational Science, 18(9).
Wang, S., et al. (2025). Decentralized clinical trials in the era of real-world evidence: A statistical perspective. Clinical and Translational Science, 18(2).

