Gene Therapy Gold Rush


The rare diseases treatment market is experiencing unprecedented transformation, projected to reach $374.4 billion by 2030 with an 11.6% CAGR. This explosive growth is driven by revolutionary gene therapy breakthroughs, enhanced regulatory frameworks, and unprecedented investment in orphan drug development. As we stand at the intersection of scientific innovation and commercial opportunity, gene therapy is emerging as the definitive solution for previously untreatable rare diseases, creating one of the most lucrative pharmaceutical market segments of the decade.
Market Landscape: The Rare Disease Renaissance
Market Size and Growth Dynamics
The global rare diseases treatment market represents one of the fastest-growing segments in pharmaceutical history:
2024 Market Value: $195.2 billion
2030 Projected Value: $374.4 billion
Growth Rate: 11.6% CAGR
Investment Surge: 340% increase in gene therapy funding since 2020
Regulatory Environment: The Foundation of Growth
The regulatory landscape has fundamentally transformed to support orphan drug development:
FDA Orphan Drug Act Impact
Since 1983, the Orphan Drug Act has provided crucial incentives:
Tax Credits: 50% credit for clinical testing costs
Market Exclusivity: 7 years of exclusive marketing rights
Fast-Track Review: Accelerated approval pathways
Waived Fees: Reduced regulatory submission costs
European Medicines Agency (EMA) Framework
The EU Orphan Medicinal Products Regulation provides:
10-Year Market Exclusivity: Extended protection period
Protocol Assistance: Scientific advice at reduced fees
Centralized Procedure: Single application across EU
Conditional Marketing Authorization: Early access pathways
Gene Therapy: The Market Disruptor
Therapeutic Modalities Driving Growth
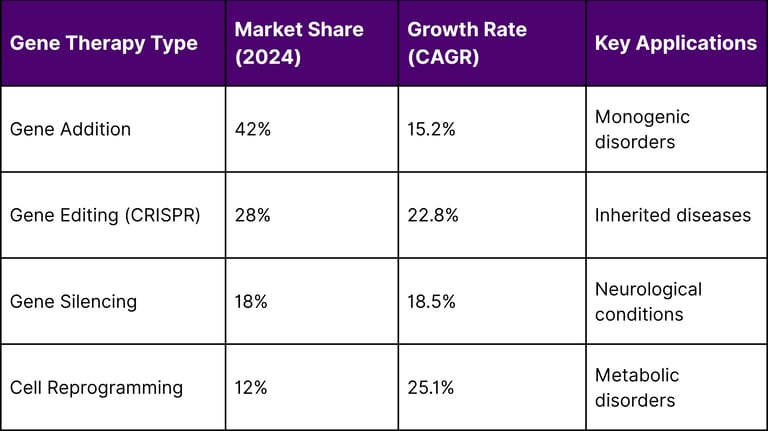
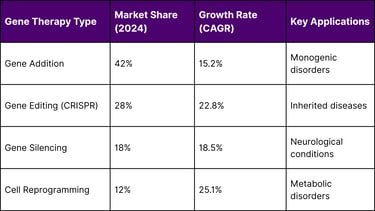
Note: These CAGR figures represent the expected annual growth rates going forward from the 2024 baseline, typically projected over a 5-10 year period.
FDA-Approved Gene Therapies: Market Leaders
Current Approvals (2020-2025):
Zolgensma (Novartis): SMA treatment, $2.1 million per dose
Luxturna (Spark Therapeutics): Inherited retinal dystrophy
Eteplirsen (Sarepta): Duchenne muscular dystrophy
Spinraza (Biogen): Spinal muscular atrophy
Elevidys (Sarepta): Gene therapy for DMD
Pipeline Analysis: The Next Wave
Late-Stage Development (2025-2027):
180+ gene therapies in Phase II/III trials
45 expected FDA submissions by 2026
$12.8 billion invested in late-stage programs
23 breakthrough therapy designations granted
Disease Area Analysis
Neurological Disorders: The Largest Segment
Market Share: 35% ($68.3 billion by 2030)
Key Conditions:
Spinal Muscular Atrophy: Market leader with 65.7% revenue share
Duchenne Muscular Dystrophy: Fastest-growing at 19.2% CAGR
Huntington's Disease: Emerging gene silencing applications
ALS: Multiple gene therapy approaches in development
Spinal Muscular Atrophy Case Study
Global Spinal Muscular Atrophy (SMA) Treatment Market dynamics:
Current Market Value: $3.2 billion (2024)
Growth Projection: 17.6% CAGR through 2030
Type 1 Dominance: 65.7% of market revenue in 2025
Treatment Paradigm: Shifted from symptomatic to curative approaches
Inherited Metabolic Disorders: High Growth Segment
Market Share: 28% ($104.8 billion by 2030)
Key Therapeutic Areas:
Lysosomal storage disorders
Glycogen storage diseases
Urea cycle disorders
Fatty acid oxidation defects
Hematological Disorders: Innovation Hub
Market Share: 22% ($82.4 billion by 2030)
Leading Applications:
Sickle cell disease gene editing
Beta-thalassemia gene therapy
Primary immunodeficiency treatments
Inherited bleeding disorders
Manufacturing and Commercialization Dynamics
Manufacturing Challenges and Solutions
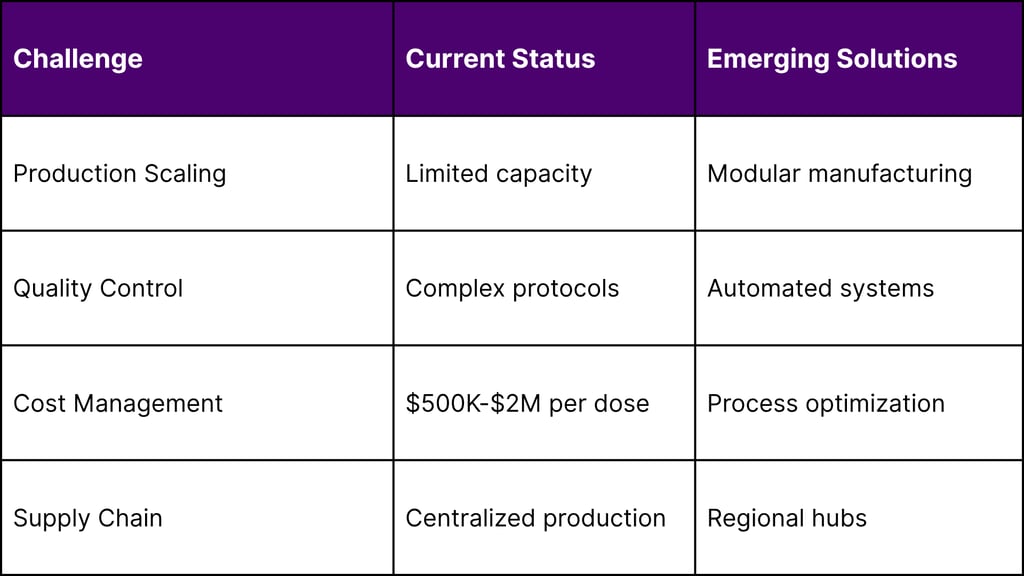
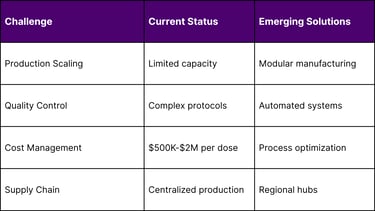
Cost-Effectiveness Models
Health Economic Considerations:
Lifetime Treatment Costs: $3-8 million for chronic care
Gene Therapy Cost: $1-3 million one-time treatment
QALY Analysis: 15-25 quality-adjusted life years gained
Payer Acceptance: Outcome-based payment models emerging
Regional Market Analysis
North America: Innovation Leader
Market Characteristics:
Market Share: 45% ($168.5 billion by 2030)
Regulatory Advantage: FDA fast-track programs
Investment Hub: 60% of global VC funding
Clinical Infrastructure: Leading trial capabilities
Key Growth Drivers:
Advanced healthcare infrastructure
Strong intellectual property protection
High healthcare spending per capita
Government research funding (NIH)
Europe: Regulatory Harmonization
Market Characteristics:
Market Share: 32% ($119.8 billion by 2030)
Regulatory Framework: EMA centralized procedures
Healthcare Integration: Cross-border collaboration
Research Excellence: Academic-industry partnerships
Asia-Pacific: Emerging Powerhouse
Market Characteristics:
Market Share: 18% ($67.4 billion by 2030)
Growth Rate: Fastest at 14.2% CAGR
Manufacturing Hub: Cost-effective production
Population Genetics: Unique disease prevalence patterns
Investment and Partnership Landscape
Venture Capital Trends
2024 Investment Highlights:
Total Gene Therapy Investment: $8.4 billion
Average Series A: $35 million
Late-Stage Funding: $120 million average
IPO Activity: 15 gene therapy companies public
Strategic Partnerships
Big Pharma Acquisitions (2023-2025):
Roche-Spark Therapeutics: $4.8 billion (2019, impact continuing)
Novartis-AveXis: $8.7 billion (2018, market expansion)
Biogen Partnerships: Multiple $1B+ collaborations
Gilead-Kite Integration: CAR-T and gene therapy synergies
Government Funding Initiatives
National Institutes of Health (NIH):
SOMATIC Program: $190 million for gene editing
COMMON Fund: $100 million for rare disease research
NCATS TRND: Therapeutic development support
FDA Critical Path: Regulatory guidance development
Competitive Landscape Analysis
Market Leaders
Novartis: Comprehensive gene therapy portfolio
Zolgensma success driving growth
Manufacturing capacity expansion
Global market penetration strategy
Pipeline diversification across disease areas
Sarepta Therapeutics: DMD focus
Multiple gene therapy approaches
Regulatory expertise in accelerated approvals
Patient advocacy partnerships
International expansion initiatives
Biogen: Neurological disorder specialization
Spinraza market leadership
Gene therapy pipeline development
Manufacturing partnerships
Digital health integration
Emerging Companies
Platform Technology Leaders:
Editas Medicine: CRISPR applications
Intellia Therapeutics: In vivo gene editing
Alnylam Pharmaceuticals: RNAi therapeutics
Solid Biosciences: Duchenne muscular dystrophy
Regulatory Pathway Evolution
FDA Guidance Development
Recent Guidance Documents (2024-2025):
Gene therapy manufacturing quality standards
Clinical trial design for rare disease applications
Long-term follow-up requirements
Combination product regulations
International Harmonization
ICH Guidelines Progress:
Quality considerations for gene therapies
Clinical development strategies
Pharmacovigilance requirements
Regulatory convergence initiatives
Market Access and Pricing Strategies
Value-Based Pricing Models
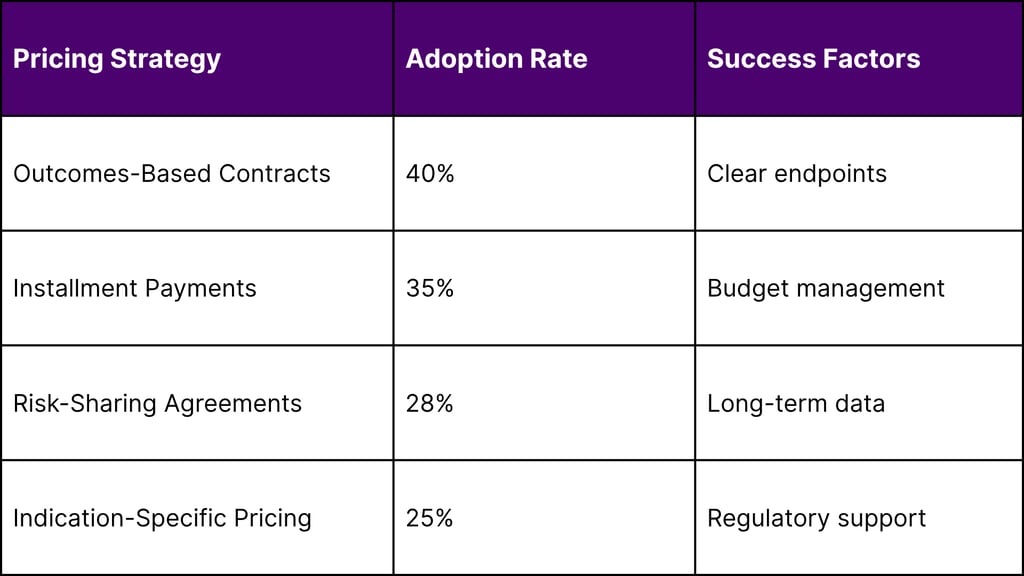
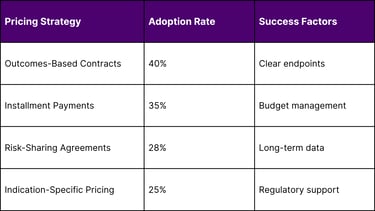
Payer Engagement Strategies
Health Technology Assessment (HTA) Considerations:
Early engagement with payer organizations
Real-world evidence generation plans
Budget impact modeling
Patient-reported outcome measures
Technology Integration and Innovation
CRISPR and Gene Editing Advances
Technical Breakthroughs:
Base Editing: Precision without double-strand breaks
Prime Editing: Targeted insertions and corrections
Epigenome Editing: Regulatory element modifications
Delivery Systems: Lipid nanoparticles and AAV vectors
Manufacturing Innovation
Process Improvements:
Continuous Manufacturing: Reduced production times
Quality by Design: Built-in quality systems
Automation: Reduced human error and costs
Analytical Methods: Real-time release testing
Challenges and Risk Mitigation
Technical Challenges
Delivery Systems:
Vector immunogenicity concerns
Tissue-specific targeting requirements
Dosing optimization needs
Long-term safety monitoring
Manufacturing Complexity:
Scale-up difficulties
Quality control requirements
Supply chain management
Regulatory compliance costs
Market Access Barriers
Pricing Pressure:
Payer budget constraints
Health technology assessment requirements
International reference pricing
Biosimilar competition emergence
Future Market Projections
Short-Term Outlook (2025-2027)
Market Drivers:
25+ gene therapy approvals expected
Manufacturing capacity doubling
Cost reduction initiatives
Payer acceptance increasing
Growth Catalysts:
Platform technology maturation
Combination therapy approaches
Digital health integration
Global market expansion
Medium-Term Projections (2027-2030)
Market Evolution:
Outpatient treatment models
Biosimilar competition beginning
Outcome-based pricing standard
Preventive applications emerging
Technology Convergence:
AI-driven drug discovery acceleration
Personalized gene therapy approaches
Multi-modal treatment strategies
Digital biomarker integration
Long-Term Vision (2030+)
Market Transformation:
Gene therapy as standard of care
Preventive genetic interventions
Global access programs established
Curative treatments for most rare diseases
Innovation Frontiers:
In vivo gene editing therapeutics
Synthetic biology applications
Tissue engineering integration
Regenerative medicine convergence
Strategic Recommendations
For Pharmaceutical Companies
Investment Priorities:
Manufacturing Capabilities: Build or acquire gene therapy production facilities
Platform Technologies: Invest in versatile delivery systems and manufacturing platforms
Regulatory Expertise: Develop specialized rare disease regulatory capabilities
Patient Access Programs: Create innovative financing and access solutions
Partnership Strategies:
Academic medical center collaborations for clinical development
Patient advocacy partnerships for disease education
Technology platform licensing agreements
International distribution partnerships
For Biotech Companies
Development Focus:
Differentiated Mechanisms: Target underserved rare disease populations
Platform Approaches: Develop technologies applicable across multiple diseases
Manufacturing Partnerships: Secure production capacity early
Regulatory Strategy: Engage agencies early for guidance
Commercialization Planning:
Specialty pharma partnerships for global reach
Direct patient support programs
Value-based contracting capabilities
International expansion strategies
For Investors
Investment Considerations:
Portfolio Diversification: Balance across disease areas and development stages
Platform vs. Single Asset: Evaluate scalability and applicability
Manufacturing Readiness: Assess production capabilities and partnerships
Regulatory Pathway: Consider approval timelines and requirements
Risk Assessment Factors:
Clinical development risks and mitigation strategies
Manufacturing complexity and scalability
Market access and reimbursement potential
Competitive landscape dynamics
Market Success Factors
Clinical Development Excellence
Critical Success Elements:
Natural History Studies: Understanding disease progression
Biomarker Development: Measuring treatment effects
Patient Stratification: Identifying optimal candidates
Long-term Follow-up: Demonstrating durability
Regulatory Strategy Optimization
Best Practices:
Early engagement with regulatory agencies
Adaptive trial design implementation
Real-world evidence generation
Global regulatory harmonization
Commercial Execution
Market Access Strategies:
Health economics and outcomes research
Payer engagement and education
Patient support programs
Healthcare provider training
Emerging Opportunities
Combination Therapies
Synergistic Approaches:
Gene therapy + small molecules
Gene editing + cell therapy
Multi-target gene therapy
Precision medicine combinations
Digital Health Integration
Technology Applications:
Remote patient monitoring
Digital biomarkers
Artificial intelligence diagnostics
Telemedicine delivery
Global Market Expansion
Emerging Markets:
Asia-Pacific regulatory development
Latin America access programs
Middle East partnership opportunities
Africa humanitarian initiatives
Risk Factors and Mitigation Strategies
Technical Risks
Manufacturing Challenges:
Risk: Production scaling difficulties
Mitigation: Modular manufacturing approaches and strategic partnerships
Safety Concerns:
Risk: Long-term adverse effects
Mitigation: Comprehensive safety monitoring and risk management plans
Commercial Risks
Market Access Barriers:
Risk: Payer resistance to high prices
Mitigation: Value-based pricing and outcome guarantees
Competitive Threats:
Risk: Multiple therapies for same indication
Mitigation: Differentiation through efficacy, safety, or convenience
Conclusion
The rare diseases treatment market stands at an unprecedented inflection point, with gene therapy driving a transformation that will reshape the pharmaceutical industry over the next decade. The convergence of scientific breakthroughs, regulatory support, and commercial opportunity creates a $374.4 billion market opportunity by 2030.
The success stories emerging from spinal muscular atrophy, Duchenne muscular dystrophy, and inherited retinal dystrophies demonstrate that gene therapy can deliver not just clinical benefits, but also substantial commercial returns. As manufacturing scales, costs decrease, and access expands, gene therapy will transition from experimental treatment to standard of care across hundreds of rare diseases.
For stakeholders across the ecosystem, the message is clear: the gene therapy gold rush is not a future opportunity—it is happening now. Organizations that can navigate the complex development landscape, build scalable manufacturing capabilities, and create innovative market access solutions will capture the most significant value in this transformative market.
The rare disease community has waited decades for effective treatments. Gene therapy is delivering on that promise while creating one of the most lucrative pharmaceutical market opportunities of our time. The question for industry participants is not whether to engage, but how quickly and effectively they can scale their participation in this revolutionary market transformation.
The convergence of unmet medical need, scientific capability, and commercial opportunity has created a perfect storm for gene therapy success in rare diseases. The gold rush is underway, and the prospectors who stake their claims today will reap the rewards for decades to come.
FAQs
1. What is driving the explosive growth of the rare disease market?
The market is projected to grow from $195.2B (2024) to $374.4B by 2030 (11.6% CAGR), driven by gene therapy breakthroughs, favorable regulatory incentives (like FDA’s Orphan Drug Act), and massive venture capital investments.
2. How is gene therapy changing the treatment landscape for rare diseases?
Gene therapy offers the potential for one-time, curative treatments targeting the genetic root cause of diseases, replacing the traditional model of lifelong symptom management.
3. Which rare diseases are seeing the biggest impact from gene therapy?
Top areas include:
Spinal Muscular Atrophy (SMA)
Duchenne Muscular Dystrophy (DMD)
Inherited retinal diseases
Beta-thalassemia and Sickle Cell Disease
These indications lead in both FDA approvals and clinical trial momentum.
4. What are the biggest regulatory advantages for orphan drugs and gene therapies?
Key benefits include:
7–10 years market exclusivity
Fast-track designations
Tax credits and waived fees
EMA centralized procedures in Europe
These reduce time-to-market and improve commercial feasibility.
5. How many gene therapies are currently approved?
As of 2025, FDA has approved several gene therapies including:
Zolgensma (SMA)
Luxturna (retinal dystrophy)
Spinraza and Elevidys (neurological disorders)
45+ new FDA submissions are expected by 2026.
6. What are the primary challenges in gene therapy commercialization?
Major hurdles include:
High manufacturing costs ($500K–$2M per dose)
Scalability of vector production
Complex regulatory compliance
Access and pricing models for one-time therapies
7. How are payers responding to the high cost of gene therapies?
Payers are adopting value-based pricing models, including:
Installment payment plans
Outcome-based contracts
Risk-sharing agreements
These align cost with clinical benefit over time.
8. Which regions are leading this market?
North America leads with 45% share due to FDA pathways and investment.
Europe follows with regulatory harmonization and public reimbursement.
Asia-Pacific is the fastest-growing region with 14.2% CAGR due to local manufacturing hubs and population-specific disease prevalence.
9. What is the investor sentiment around gene therapy?
Very bullish. In 2024 alone:
$8.4B was invested globally
Late-stage Series B/C rounds averaged $120M
IPOs surged with 15 gene therapy companies going public
10. What’s the long-term outlook for gene therapy in rare diseases?
By 2030+, gene therapy is expected to become:
The standard of care for many monogenic diseases
A curative solution replacing chronic therapies
A cornerstone of personalized, precision medicine
References
U.S. Food and Drug Administration (FDA) – Drug Approvals and Databases
European Medicines Agency (EMA) – Regulatory Guidelines and Approvals
National Institutes of Health (NIH) – Research Portfolio Online Reporting Tools (RePORT)
World Health Organization (WHO) – Rare Disease Statistics and Global Health Estimates
Peer-Reviewed Clinical Research Publications

