Gene Therapy Revolution in Neurometabolic Disorders

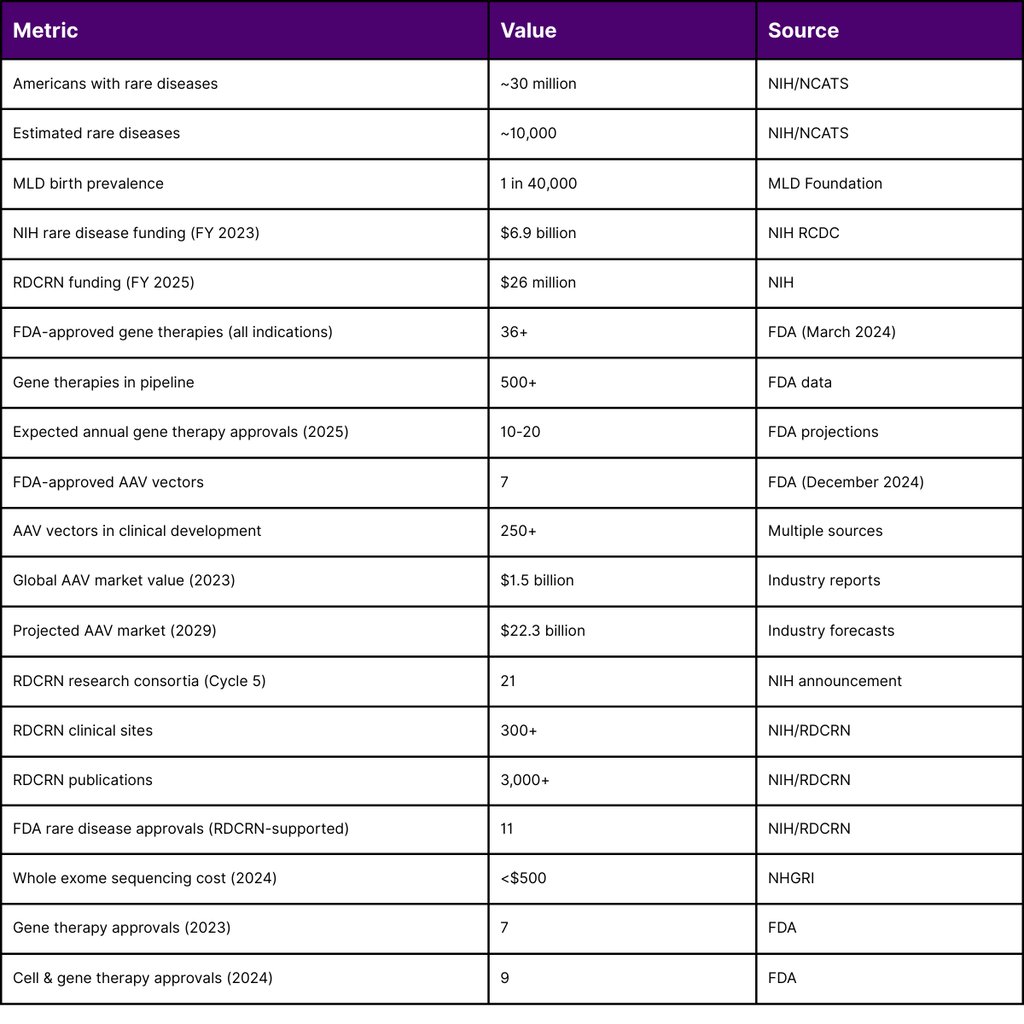
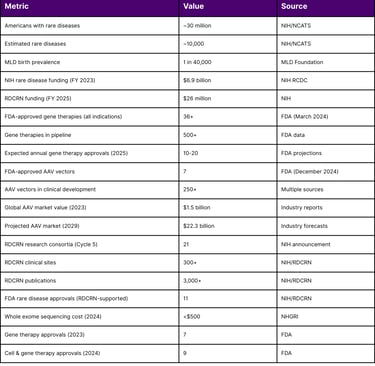
Neurometabolic disorders represent a group of rare genetic conditions affecting the nervous system's metabolic function, collectively impacting approximately 1 in 40,000 births. While each individual disorder affects a small patient population, the cumulative impact is substantial approximately 30 million Americans live with one of an estimated 10,000 rare diseases. The landscape of treatment for these devastating conditions has undergone a dramatic transformation in 2024-2025, with gene therapy emerging as a viable therapeutic option for several previously untreatable neurometabolic disorders.
The Current State of FDA-Approved Gene Therapies for Neurometabolic Disorders
As of December 2024, the U.S. Food and Drug Administration (FDA) has approved several groundbreaking gene therapies specifically targeting neurometabolic disorders. These approvals represent a paradigm shift in how the medical community approaches rare genetic diseases affecting the nervous system.
Recent FDA Approvals
LENMELDY (atidarsagene autotemcel) - Approved in March 2024, LENMELDY became the first gene therapy approved in the United States for children with early-onset metachromatic leukodystrophy (MLD). This one-time, autologous hematopoietic stem cell (HSC) gene therapy targets pre-symptomatic late infantile, pre-symptomatic early juvenile, and early symptomatic early juvenile MLD. The therapy works by modifying a patient's own stem cells to carry a functional ARSA gene, enabling the body to break down harmful sulfatides that accumulate in the nervous system.
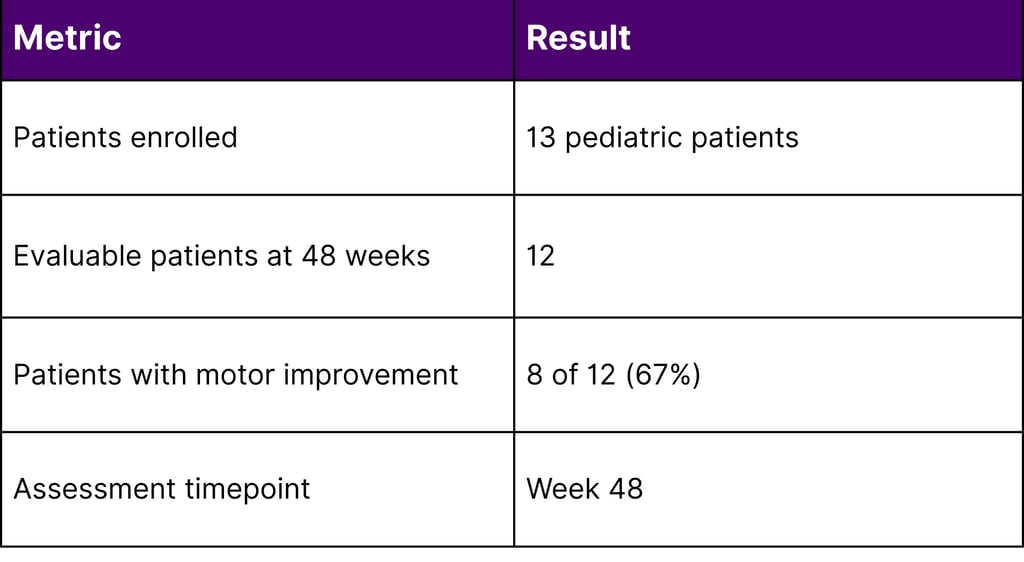
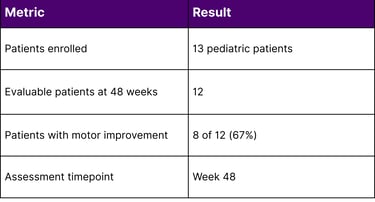
KEBILIDI (eladocagene exuparvovec-tneq) - Approved in November 2024, KEBILIDI represents a significant milestone as the first gene therapy for aromatic L-amino acid decarboxylase (AADC) deficiency and the first FDA-approved gene therapy delivered directly into the brain. AADC deficiency affects the body's ability to produce essential neurotransmitters, resulting in severe motor dysfunction, developmental delays, and cognitive impairment. In clinical trials involving 13 pediatric patients, 8 of 12 evaluable patients (67%) showed improvements in gross motor function at 48 weeks following treatment.
SKYSONA (elivaldogene autotemcel) - Approved in 2022, SKYSONA treats early cerebral adrenoleukodystrophy (CALD) in boys who are not yet experiencing symptoms. This autologous HSC gene therapy has demonstrated the ability to slow disease progression in this rapidly degenerative condition.
The Viral Vector Landscape: AAV and Lentiviral Platforms Dominate
The gene therapy approvals for neurometabolic disorders primarily utilize viral vector delivery systems, specifically adeno-associated virus (AAV) and lentiviral vectors. These platforms have proven track records of safety and efficacy in clinical applications.
AAV Vector Therapies
AAV vectors have become the predominant delivery platform for in vivo gene therapy, with KEBILIDI representing the first AAV gene therapy delivered through MRI-guided intracranial administration. As of December 2024, there are seven FDA-approved AAV-based gene therapies across all indications, with over 250 AAV vector gene therapies in clinical development.
The global market value of AAV therapies reached $1.5 billion in 2023 and is projected to reach $22.3 billion by 2029. In 2024 alone, the FDA approved BEQVEZ (fidanacogene elaparvovec) for hemophilia B, demonstrating the continued viability of the AAV platform.
Lentiviral Vector Therapies
Lentiviral vectors dominate the ex vivo gene therapy space for neurometabolic disorders. Both LENMELDY and SKYSONA utilize lentiviral vectors to modify patients' hematopoietic stem cells ex vivo before reinfusion. This approach offers the advantage of permanent genetic correction in multipotent cells, ensuring long-term therapeutic benefit as these cells differentiate and proliferate.
The Clinical Pipeline: Expanding Therapeutic Options
According to the FDA's Office of Orphan Products Development and data from ClinicalTrials.gov, the pipeline for neurometabolic gene therapies continues to expand significantly in 2024-2025.
Key Pipeline Developments
Methylmalonic Acidemia (MMA) - The National Institutes of Health's National Center for Advancing Translational Sciences (NCATS) and National Human Genome Research Institute (NHGRI) announced in November 2024 a collaboration to advance a gene therapy for MMA into clinical trials, expected to begin in fall 2025. This development demonstrates the federal government's commitment to advancing rare disease therapies even when private sector support wanes.
Mucopolysaccharidosis IIIA (Sanfilippo A Syndrome) - In December 2024, UX111 (rivunatpagene miziparvovec), an AAV gene therapy for MPS IIIA, advanced to NDA/BLA filing status, representing another neurometabolic disorder approaching potential FDA approval.
Neurological Disorder Focus
Neurological disorders, including neurometabolic conditions, are emerging as a major frontier in gene therapy development. Several key regulatory milestones were achieved in 2024-2025:
MavriX Bio received FDA clearance for a first-in-human Phase 1 study of an AAV gene therapy for Angelman syndrome
MeiraGTx's AAV gene therapy for Parkinson's disease received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA
Regulatory Environment and Government Support
The regulatory landscape has become increasingly supportive of rare disease gene therapy development, with multiple expedited pathways available.
FDA Accelerated Approval Pathways
The FDA has established several mechanisms to expedite gene therapy development for rare diseases:
Breakthrough Therapy Designation - Granted to multiple gene therapy candidates targeting neurometabolic disorders
Regenerative Medicine Advanced Therapy (RMAT) Designation - Intended to expedite development and review of therapies addressing serious conditions
Orphan Drug Designation - Over 25 orphan drugs for neurometabolic disorders have been approved since 2020
Platform Technology Designation
In a significant regulatory evolution, the FDA granted one of its first "platform technology designations" for a viral vector (Sarepta's viral vector for muscular dystrophy), signaling a move toward streamlining the development pathway for validated platforms. This approach recognizes that established vector platforms with proven safety profiles may require less extensive characterization for new therapeutic applications.
NIH Funding and Research Investment
Federal investment in rare disease research has grown substantially, reflecting governmental commitment to addressing unmet medical needs in neurometabolic disorders.
Rare Diseases Clinical Research Network (RDCRN)
In fiscal year 2025, the NIH awarded approximately $26 million to establish the fifth cycle of funding for the RDCRN. This national network brings together scientists, clinicians, patients, families, and patient advocates to study a wide range of rare diseases. The network currently consists of 21 research consortia studying at least three different rare diseases each. Since its establishment in 2003, the RDCRN has:
Supported hundreds of studies at over 300 clinical sites worldwide
Generated over 3,000 publications
Contributed to the approval of 11 FDA treatments for rare diseases
Categorical Spending on Rare Diseases
According to the NIH's Research, Condition, and Disease Categorization (RCDC) system, rare disease funding reached approximately $6.9 billion in fiscal year 2023, with FY 2024 estimates showing continued growth. The NIH allocated funding for rare disease research across multiple institutes through various mechanisms:
Preclinical Proof of Concept Studies for Rare Diseases (R21)
Clinical Trial Readiness for Rare Diseases (R21)
Pilot Projects Investigating Understudied Proteins Associated with Rare Diseases (R03)
Manufacturing and Scalability Challenges
Despite remarkable clinical progress, gene therapy manufacturing presents significant challenges that impact patient access and treatment sustainability.
Production Capacity
The production of high-dose gene therapies cannot match the pace of traditional pharmaceutical manufacturing due to complex processes. AAV gene therapy manufacturing requires:
Sophisticated analytical tools
Specialized production facilities
Stringent quality control measures
Significant time investment per patient dose
Treatment Infrastructure
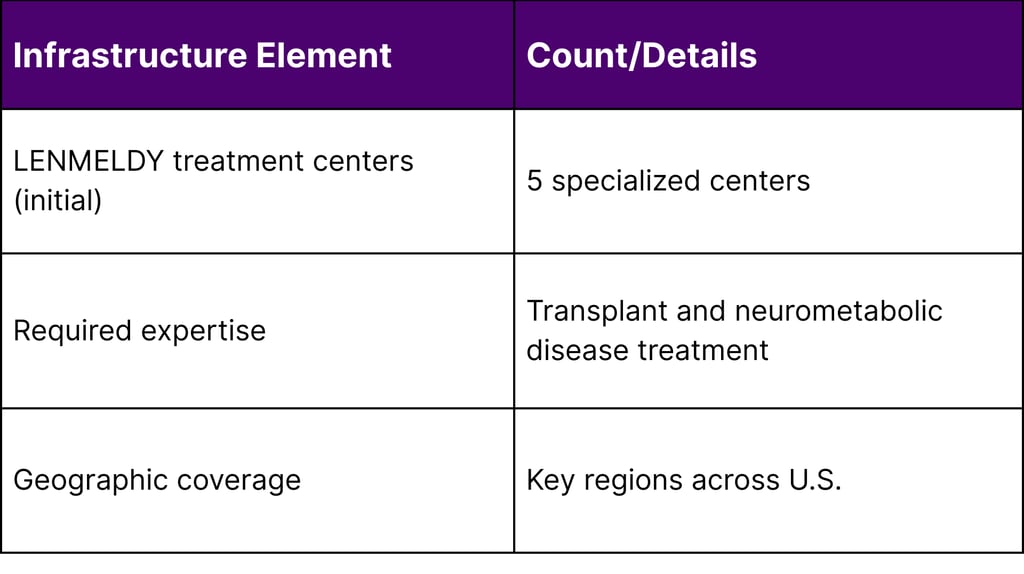
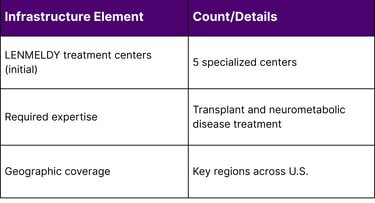
Five specialized treatment centers with expertise in transplant and neurometabolic diseases were activated to administer LENMELDY in 2024. This limited infrastructure presents logistical challenges for families who may need to travel significant distances for treatment. Many payers and healthcare systems now offer travel concierge support and expense reimbursement to address this barrier.
Economic Considerations and Patient Access
The cost of gene therapies for neurometabolic disorders represents a significant challenge for healthcare systems and payers.
Pricing Landscape
Recent approvals illustrate the high cost of these one-time treatments:
LENMELDY: $4.25 million per patient
HEMGENIX (for hemophilia B): $3.5 million per patient
ZOLGENSMA (for spinal muscular atrophy): Approximately $2.1 million per patient
The Institute for Clinical and Economic Review (ICER) determined that LENMELDY's health benefit price benchmark should range between $2.29 million and $3.94 million, noting that the actual price exceeds the upper bound of cost-effectiveness estimates by $310,000.
Insurance and Reimbursement
Infants with MLD and other neurometabolic disorders typically qualify for government assistance, making state Medicaid programs a critical component of the reimbursement landscape. Healthcare systems are developing innovative financing mechanisms, including:
Stop-loss insurance arrangements
Value-based pricing models
Installment payment structures
Outcomes-based reimbursement agreements
Early Detection and Newborn Screening
The effectiveness of gene therapy for neurometabolic disorders often depends on early intervention before irreversible neurological damage occurs.
Newborn Screening Expansion
A collaborative multi-stakeholder group is in final stages of proposing MLD for addition to the U.S. Recommended Uniform Screening Panel (RUSP). If approved, this would enable:
Earlier diagnosis before symptom onset
Expanded treatment eligibility
Improved long-term outcomes
Greater cost-effectiveness of therapy
Diagnostic Advancements
Genetic sequencing costs have decreased dramatically, from $5,000 in 2012 to under $500 in 2024, making diagnostic testing more accessible for neurometabolic disorder identification. Whole exome sequencing has significantly improved early detection rates, enabling timely intervention.
Global Perspective and International Developments
Gene therapy development for neurometabolic disorders is advancing globally, with regulatory approvals and clinical programs emerging in multiple regions.
International Approvals
Canada: BEQVEZ (fidanacogene elaparvovec) was approved in January 2024 for hemophilia B
Europe: LENMELDY received marketing authorization as Libmeldy before U.S. approval
China: BBM-H901, an AAV-delivered treatment for hemophilia B, became the first gene therapy of its kind approved in China in 2025
Japan: In October 2025, OTL-200 (LENMELDY) was granted Orphan Regenerative Medicine Product Designation for early-onset MLD
Safety Considerations and Adverse Events
While gene therapies have demonstrated generally favorable safety profiles, certain adverse events require ongoing monitoring and investigation.
Identified Safety Concerns
Immunological Responses: High AAV doses (>1 × 10^14 vg/kg) can trigger substantial immunological toxicity
Thrombotic Microangiopathy (TMA): Observed across several trials using high doses of AAV9 capsid
Transgene Overexpression: Can lead to complications such as thrombosis in hemophilia trials
Pre-existing Neutralizing Antibodies: Can limit efficacy of AAV vector therapies
Vector Shedding: AAV is shed in bodily fluids for durations dependent on vector dose
Common adverse reactions for LENMELDY include stomatitis, thrombocytopenia, and neutropenia, typical of myeloablative conditioning regimens required for HSC gene therapy.
Future Directions and Emerging Technologies
The gene therapy field is evolving rapidly with several technological innovations on the horizon.
Engineered AAV Capsids
The use of engineered capsids is increasing substantially, with 39 clinical trials now utilizing 15 unique novel capsids, compared to just 20 trials two years prior. These next-generation capsids aim to improve:
Tissue-specific targeting
Reduced immunogenicity
Enhanced transduction efficiency
Ability to evade pre-existing immunity
Alternative Delivery Approaches
While viral vectors dominate current approvals, research continues into alternative delivery mechanisms, though these remain primarily in preclinical stages for neurometabolic applications.
CRISPR-Based Therapies
The approval of Casgevy (exagamglogene autotemcel) in 2024 for sickle cell disease and beta-thalassemia demonstrated the first CRISPR-based gene therapy approval in the United States. While not yet applied to neurometabolic disorders, this technology represents a potential future avenue for genetic correction.
Industry Trends and Investment
The gene therapy sector has experienced significant investment activity despite economic headwinds, reflecting confidence in the therapeutic modality's long-term potential.
M&A Activity
Major pharmaceutical companies made substantial investments in gene therapy platforms in 2024:
Novartis acquired Kate Therapeutics for $1.1 billion (November 2024)
Roche acquired Poseida Therapeutics for $1.5 billion (Q4 2024)
These acquisitions underscore pharmaceutical industry recognition that gene therapy platforms represent valuable long-term assets.
Early-Stage Funding
Seed and Series A financing for gene therapy companies reached $609.2 million in Q4 2024, up 26% from Q3, indicating continued investor confidence in early-stage innovation.
Patient Advocacy and Collaborative Research
Patient advocacy groups play a critical role in advancing gene therapy research and development for neurometabolic disorders.
Coalition of Patient Advocacy Groups (CPAG)
The RDCRN includes patient advocacy group partners who participate in:
Individual consortium research activities
Network-wide governance through the CPAG Steering Committee
Research prioritization and design
Patient recruitment and engagement
Dissemination of research findings
Foundation Support
Private foundations and advocacy organizations provide crucial funding that complements federal research investments. These organizations often fund pilot studies, natural history studies, and biomarker development that enable future clinical trials.
Statistical Overview
Key Statistics from Government Sources
Treatment Center and Infrastructure
Clinical Outcomes - KEBILIDI Trial
Conclusion
The treatment landscape for neurometabolic disorders has been fundamentally transformed by gene therapy advances in 2024-2025. With multiple FDA approvals, robust pipeline development, and substantial government investment through the NIH, patients with previously untreatable conditions now have therapeutic options. The field continues to mature with improvements in manufacturing, regulatory frameworks, and understanding of safety profiles.
However, significant challenges remain. Manufacturing scalability, high treatment costs, limited treatment center infrastructure, and the need for early detection through expanded newborn screening all require continued attention. The collaborative efforts of federal agencies, academic researchers, industry partners, and patient advocacy organizations will be essential to ensuring these groundbreaking therapies reach all eligible patients.
As the gene therapy pipeline continues to expand with over 250 AAV vectors alone in clinical development the coming years promise additional treatment options for neurometabolic disorders. The regulatory precedents established with LENMELDY, KEBILIDI, and SKYSONA, combined with innovations like platform technology designations, will facilitate more efficient pathways for future therapies.
The convergence of technological innovation, regulatory support, and sustained investment positions gene therapy as a transformative treatment paradigm for neurometabolic disorders, offering hope to patients and families affected by these devastating conditions.
Frequently Asked Questions (FAQ)
Q1: What are neurometabolic disorders? A: Neurometabolic disorders are rare genetic conditions that affect the nervous system's metabolic function. They result from genetic mutations that impair the body's ability to process certain substances, leading to toxic accumulation or deficiencies that damage the nervous system. Examples include metachromatic leukodystrophy (MLD), aromatic L-amino acid decarboxylase (AADC) deficiency, and cerebral adrenoleukodystrophy (CALD).
Q2: How do gene therapies work for neurometabolic disorders? A: Gene therapies work by delivering a functional copy of the defective gene or by modifying a patient's own cells to produce the missing or deficient protein. For ex vivo therapies like LENMELDY, stem cells are removed from the patient, genetically modified in the laboratory, and then returned to the patient. For in vivo therapies like KEBILIDI, the therapeutic gene is delivered directly to the target tissue using a viral vector.
Q3: Why are viral vectors used in gene therapy? A: Viral vectors, particularly AAV and lentiviral vectors, are used because they efficiently deliver genetic material into cells. These vectors have been modified to remove disease-causing viral genes while retaining their ability to enter cells and deliver therapeutic genes. AAV vectors are especially useful because they infect a wide range of cell types, have low immunogenicity, and do not integrate into the genome in most cases.
Q4: Are these gene therapies curative? A: Gene therapies for neurometabolic disorders are designed to be one-time treatments that provide long-term benefit by addressing the underlying genetic cause. However, they are most effective when administered before irreversible neurological damage occurs. Early intervention through newborn screening and presymptomatic diagnosis is crucial for optimal outcomes.
Q5: What is the role of the NIH in gene therapy development? A: The NIH plays multiple critical roles: funding basic and translational research through institutes like NCATS and NHGRI, supporting clinical research networks like the RDCRN, conducting natural history studies, developing biomarkers for clinical trials, and in some cases, directly advancing therapies toward clinical trials when private sector support is unavailable.
Q6: Why do gene therapies cost millions of dollars? A: The high cost reflects several factors: extensive research and development spanning many years, complex and specialized manufacturing processes, small patient populations limiting economies of scale, regulatory compliance requirements, clinical trial expenses, and the one-time curative nature of the treatment compared to lifetime costs of conventional therapies. Manufacturing each patient's dose requires significant time and specialized facilities.
Q7: How many gene therapies have been approved for neurometabolic disorders? A: As of December 2024, the FDA has approved three gene therapies specifically for neurometabolic disorders: LENMELDY for MLD, KEBILIDI for AADC deficiency, and SKYSONA for CALD. Additional therapies are in late-stage clinical development and regulatory review.
Q8: What is newborn screening and why is it important? A: Newborn screening involves testing babies shortly after birth for genetic disorders that can be treated if caught early. For gene therapy to be most effective in neurometabolic disorders, it must be administered before symptoms appear and irreversible damage occurs. Expanded newborn screening for conditions like MLD would enable earlier diagnosis and treatment, improving outcomes and cost-effectiveness.
Q9: Are there risks associated with gene therapy? A: Yes, gene therapies can have risks including immune responses to the viral vector, adverse reactions to conditioning regimens (for ex vivo therapies), potential for unintended genetic changes, and manufacturing-related concerns. However, clinical trials have demonstrated generally favorable safety profiles when therapies are properly administered with appropriate patient monitoring.
Q10: How can patients access these gene therapies? A: Patients must first have a confirmed diagnosis through genetic testing. Treatment is available only at specialized qualified treatment centers with expertise in gene therapy administration and neurometabolic diseases. Insurance coverage typically requires prior authorization, though patients with rare diseases often qualify for government assistance through Medicaid programs. Patient assistance programs are available through manufacturers to help navigate insurance and access issues.
References
American Society of Gene & Cell Therapy. (2024). Gene, cell, & RNA therapy landscape report Q4 2024.
Centers for Disease Control and Prevention, National Center for Health Statistics. (2024). Disease burden data.
Kayki-Mutlu, G., Rathfon, A., & Michel, M. C. (2025). A year in pharmacology: New drugs approved by the US Food and Drug Administration in 2024. Naunyn-Schmiedeberg's Archives of Pharmacology.
MLD Foundation. (n.d.). What is MLD?
National Center for Advancing Translational Sciences. (2024, November 19). New path for a gene therapy trial at NIH for a rare metabolic disease. National Institutes of Health.
National Center for Advancing Translational Sciences. (2024). Preclinical proof of concept studies for rare diseases (R21 clinical trial not allowed) [Funding opportunity RFA-TR-25-002]. National Institutes of Health.
National Center for Advancing Translational Sciences. (2024). Clinical trial readiness for rare diseases, disorders, and syndromes (R21 clinical trial not allowed) [Program announcement PAR-25-450]. National Institutes of Health.
National Human Genome Research Institute. (2024). Whole exome sequencing costs. National Institutes of Health.
National Institutes of Health. (2024). Pilot projects investigating understudied proteins associated with rare diseases (R03 clinical trial not allowed) [Program announcement PAR-25-122].
National Institutes of Health. (2025, June 17). Funding for various research, condition, and disease categories (RCDC). NIH RePORT.
National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases. (2025, February 7). NIAMS update, Issue 6, 2024.
PackGene Biotech. (2025, November 3). Advances in cell and gene therapy and the evolving AAV landscape in 2025 H1.
Rare Diseases Clinical Research Network. (2025, October). NIH announces funding to establish and strengthen rare disease research groups.
Samulski, R. J., & Muzyczka, N. (2014). AAV-mediated gene therapy for research and therapeutic purposes. Annual Review of Virology, 1, 427-451.
U.S. Food and Drug Administration. (2024, November 14). FDA approves novel gene therapy for rare neurometabolic disorder [Press release].
U.S. Food and Drug Administration. (2024, November 26). Approved cellular and gene therapy products.
U.S. Food and Drug Administration. (n.d.). Funding opportunities for rare diseases at FDA.

