Generative AI Meets Biotech: Re-Imagining Drug Design from Target to Trial


The pharmaceutical industry faces an unprecedented challenge: developing a new drug costs approximately $879.3 million when accounting for failures and capital costs, with the clinical phase lasting an average of around 95 months compared to 31 months for the non-clinical phase. Against this backdrop, generative artificial intelligence—specifically deep learning architectures including Generative Adversarial Networks (GANs), Variational Autoencoders (VAEs), and transformer-based models—is emerging as a transformative force in drug discovery and development.
Artificial intelligence is revolutionizing drug discovery by enhancing precision, reducing timelines and costs, and enabling AI-driven computer-aided drug design. This article examines how generative AI models are fundamentally reshaping the drug development paradigm from molecular target identification through clinical trial optimization, offering pharmaceutical and biotech companies unprecedented opportunities to accelerate innovation.
The Drug Development Challenge: Cost, Time, and Complexity
The Financial Reality
The estimated mean cost of developing a new drug is approximately $172.7 million in out-of-pocket expenses, increasing to $515.8 million when cost of failures is included, and reaching $879.3 million when both costs of failures and capital are factored in. These costs vary significantly by therapeutic area, ranging from $378.7 million for anti-infectives to $1756.2 million for pain and anesthesia drugs.
The pharmaceutical industry has responded by increasing R&D intensity from 11.9% to 17.7% from 2008 to 2019, with large pharmaceutical companies increasing from 16.6% to 19.3%.
The Timeline Challenge
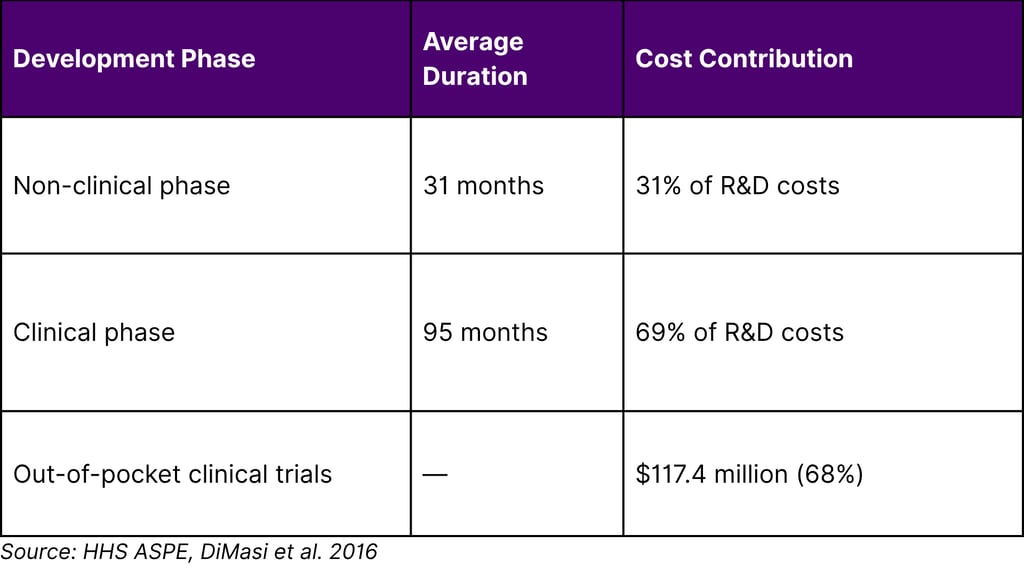
In drug development, the clinical phase lasts an average of around 95 months compared to 31 months for the non-clinical phase and accounts for 69 percent of overall R&D costs. Clinical trials comprise the largest portion of overall drug development costs at $117.4 million, accounting for around 68 percent of out-of-pocket R&D expenditures.
Table 1: Drug Development Cost Breakdown
Cost Drivers and Barriers
The factors that contribute the most to costs across all trial phases include Clinical Procedure Costs (15 to 22 percent), Administrative Staff Costs (11 to 29 percent), Site Monitoring Costs (nine to 14 percent), Site Retention Costs (nine to 16 percent), and Central Laboratory Costs (four to 12 percent).
The major obstacles to conducting clinical trials in the United States include high financial cost, lengthy time frames, difficulties in recruitment and retention of participants, insufficiencies in the clinical research workforce, drug sponsor-imposed barriers, regulatory and administrative barriers, the disconnect between clinical research and medical care.
These challenges create an urgent need for innovation in drug development processes.
Understanding Generative AI: A Paradigm Shift in Drug Discovery
Defining Generative AI
Artificial Intelligence (AI) and Machine Learning (ML) can be described as a branch of computer science, statistics, and engineering that uses algorithms or models to perform tasks and exhibit behaviors such as learning, making decisions, and making predictions. ML is considered a subset of AI that allows models to be developed by training algorithms through analysis of data, without models being explicitly programmed.
Generative AI represents a specialized class of machine learning that creates entirely new data—in this context, novel molecular structures—rather than simply analyzing existing information.
The Power of Generative Models
From the generation of original texts, images, and videos, to the scratching of novel molecular structures, the creativity of deep learning generative models exhibits the height machine intelligence can achieve. Promising and compelling outcomes including the identification of DDR1 kinase inhibitors within 21 days using deep learning generative models may indicate that we are probably at the corner of a new era.
Core Generative AI Architectures
1. Recurrent Neural Networks (RNNs)
Recurrent neural networks (RNNs) are state-of-the-art generative models focused on for their fundamental architectures as well as their applications in de novo drug design. RNNs excel at processing sequential data, making them ideal for molecular representations like SMILES strings.
2. Variational Autoencoders (VAEs)
Variational autoencoders (VAEs) represent cutting-edge generative architectures used for compound generation in drug discovery. VAEs compress molecular representations into lower-dimensional latent spaces, enabling efficient exploration of chemical space and property prediction.
3. Adversarial Autoencoders (AAEs)
Adversarial autoencoders (AAEs) combine the benefits of autoencoders with adversarial training for improved molecular generation.
4. Generative Adversarial Networks (GANs)
Generative adversarial networks (GANs) are among the state-of-the-art generative models with detailed discussions on utilizing cutting-edge generative architectures for compound generation. GANs employ a dual-network architecture where a generator creates candidate molecules while a discriminator evaluates their validity, producing increasingly sophisticated molecular designs through adversarial training.
Generative AI Across the Drug Development Lifecycle
Stage 1: Drug Discovery and Target Identification
Computational technologies generate vast amounts of data on drug-like properties of chemical compounds which can bind to therapeutic targets. At the same time, computational technologies help to develop 3D structures of the therapeutic targets.
AI/ML could accelerate advances in de novo drug design, fundamentally changing how researchers identify and validate therapeutic targets.
Applications in Drug Discovery:
AI/ML technologies are being used for drug target identification, selection, and prioritization, as well as compound screening and design.
One significant protein-based drug target class is G-protein coupled receptor (GPCR). Drugs targeting the GPCR class contain angiotensin receptor blockers, β-blockers, opioid agonists, and histamine receptor blockers.
Stage 2: De Novo Molecular Design
There are two main ways to discover or design small drug molecules. The first involves fine-tuning existing molecules or commercially successful drugs through quantitative structure-activity relationships and virtual screening. The second approach involves generating new molecules through de novo drug design or inverse quantitative structure-activity relationship.
Generative artificial intelligence in drug discovery has delivered promising results, with the ability to generate entirely new data such as novel chemical molecules.
The De Novo Design Process:
Putin et al. explored a DNN architecture called the reinforced adversarial neural computer (RANC) based on RL for de novo design of small organic molecules. This platform was trained with molecules represented as SMILES strings and then generated molecules with predefined chemical descriptors in terms of MW, logP, and topological polar surface area (TPSA).
Stage 3: Property Prediction and Optimization
Numerous criteria, including ADMET (absorption, distribution, metabolism, excretion, and toxicity) and synthesis feasibility, must be satisfied by each potential drug candidate. Therefore, predicting molecular characteristics with high precision is crucial in the drug development process.
Different AI-based tools can be used to predict physicochemical properties. For example, ML uses large data sets produced during compound optimization done previously to train the program.
Toxicity Prediction:
Developed using an ML-based approach, eToxPred was applied to estimate the toxicity and synthesis feasibility of small organic molecules and showed accuracy as high as 72%.
Stage 4: Synthesis Planning
DNN focuses on rules of organic chemistry and retrosynthesis which, with the aid of Monte-Carlo tree searches and symbolic AI, help in reaction prediction and the process of drug discovery and design, which is much faster than traditional methods.
Coley et al. developed a framework in which a rigid forward reaction template was applied to a group of reactants to synthesize chemically feasible products with a significant rate of reaction.
Stage 5: Clinical Trial Design and Optimization
AI/ML technologies are increasingly being used in clinical research for recruitment, selection of trial participants.
Predictive models can be used to enrich clinical trials, improving patient selection and trial efficiency.
Potential Cost Reductions:
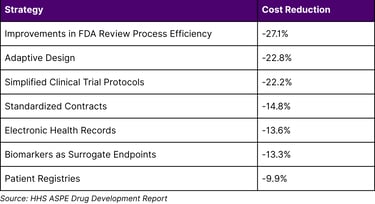
The study found that the strategy with the largest expected impact on overall development costs across all therapeutic areas was Improvements in FDA Review Process Efficiency and Interactions (-27.1 percent), followed by Adaptive Design (-22.8 percent), and implementation of Simplified Clinical Trial Protocols and Reduced Amendments (-22.2 percent).
FDA Regulatory Framework and Industry Adoption
FDA Recognition and Engagement
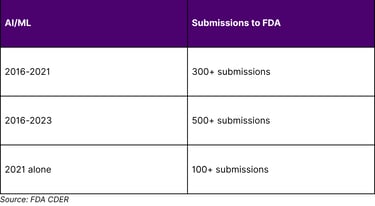
FDA recognizes the increased use of AI throughout the drug product life cycle and across a range of therapeutic areas. In fact, CDER has seen a significant increase in the number of drug application submissions using AI components over the past few years.
CDER's experience includes over 500 submissions with AI components from 2016 to 2023. FDA has seen a significant increase in the number of drug and biologic application submissions using AI/ML components over the past few years, with more than 100 submissions reported in 2021.
Figure 1: FDA AI Submission Growth

Regulatory Framework Development
The CDER AI Council was established in 2024 to provide oversight, coordination, and consolidation of CDER activities around AI use.
The content of the draft guidance was informed by feedback received in December 2022 as part of an expert workshop convened by the Duke Margolis Institute for Health Policy on behalf of CDER/FDA, over 800 comments received from external parties on the discussion paper published in May 2023 on AI use in drug development, and a hybrid public workshop for interested parties held on August 6, 2024.
Key FDA Publications:
FDA published draft guidance in 2025 titled "Considerations for the Use of Artificial Intelligence to Support Regulatory Decision-Making for Drug and Biological Products".
The FDA issued draft guidance to provide recommendations on the use of artificial intelligence intended to support a regulatory decision about a drug or biological product's safety, effectiveness or quality. This is the first guidance the agency has issued on the use of AI for the development of drug and biological products.
FDA's Risk-Based Approach
A key aspect to the appropriate application of AI modeling in drug development and regulatory evaluation is ensuring model credibility—trust in the performance of an AI model for a particular context of use. This guidance provides a risk-based framework for sponsors to assess and establish the credibility of an AI model for a particular context of use.
FDA plans on developing and adapting a flexible risk-based regulatory framework that will promote innovation and protect patient safety.
Real-World Applications and Industry Impact
Pharmaceutical Industry Adoption
Several biopharmaceutical companies, such as Bayer, Roche, and Pfizer, have teamed up with IT companies to develop a platform for the discovery of therapies in areas such as immuno-oncology and cardiovascular diseases.
As of 2024, there are no on-market medications that have been developed using an AI-first pipeline, though the integration of AI in drug R&D is poised to accelerate.
Technology Company Involvement
Looking ahead, the integration of AI in drug R&D is poised to accelerate, driven by advancements from leading tech companies. NVIDIA's powerful GPUs and AI frameworks are enabling faster and more efficient generative drug discovery processes. Google Health is leveraging its expertise in data analytics and ML to enhance predictive modeling and patient data analysis.
AlphaFold and Protein Structure Prediction
The 2024 Nobel Prize in Chemistry was awarded to David Baker, Demis Hassabis, and John Jumper for their groundbreaking work in using AI to predict protein structures and design functional proteins. The development of the AlphaFold model has solved a long-standing challenge in biology by accurately predicting the complex structures of proteins.
Clinical Pharmacology Community Perspectives
Survey Insights
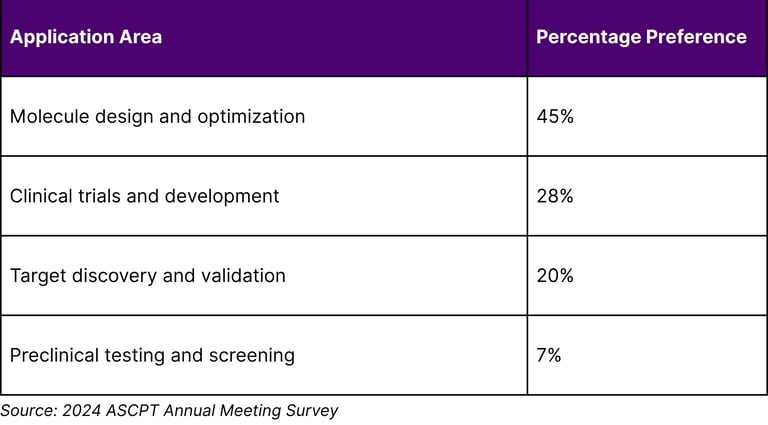
A survey at the 2024 American Society of Clinical Pharmacology and Therapeutics Annual Meeting revealed that regarding AI's future impact in the next 5–10 years, 45% highlighted a preference for its application in molecule design and optimization, followed by clinical trials and development (28%), target discovery and validation (20%), and preclinical testing and screening (7%).
Table 2: Clinical Pharmacology Community AI Priorities (Next 5-10 Years)
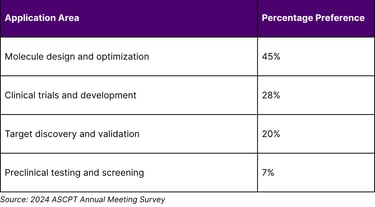
The results highlight the current familiarity, usage, and perceptions of AI among the clinical pharmacology community, indicating a strong interest and optimism about AI's role in the future of drug development.
Technical Capabilities and Limitations
Current Capabilities
Fast computational methodologies have been developed using computational resources and technologies. One example is structure-based virtual screening using Giga scale chemical spaces where technology screened millions to billions of compounds.
AI-based technologies have been helping to speed up the drug discovery and development process. AI-derived drug molecules show a chemical space similar to previously published drugs.
Data and Infrastructure Requirements
DL models and big data enhance the processing of unstructured data, which helps with more potent QSAR model formation and provides a comprehensive interpretation of the drug discovery process.
Several online prediction platforms have been developed using AI/ML algorithms for drug molecule structure prediction, understanding the drug ability and characteristics of the molecule that help drug discovery and development.
Validation Challenges
Our revision of AI-generated text required the application of major edits and corrections. This is a huge problem with current AI tools and represents a key difference with respect to other computational tools which are focused on providing reliable references for required information.
This highlights the ongoing need for human expertise and validation in AI-assisted drug discovery.
Economic Implications and Cost-Benefit Analysis
Development Cost Estimates
Bringing a new drug to market is an expensive and time-consuming endeavor, with the average cost being estimated at around $2.5 billion, though government data suggests $879.3 million when both costs of failures and capital are included.
Potential Cost Reduction Strategies
The HHS ASPE analysis identified several strategies with significant cost-reduction potential:
Table 3: Impact of Clinical Trial Strategies on Development Costs

Therapeutic Area Variations
The therapeutic area with the highest clinical research burden across all phases is respiratory system ($115.3 million) followed by pain and anesthesia ($105.4 million).
Challenges and Considerations
Technical Challenges
Data Quality and Availability:
One of the biggest challenges is screening the vast number of potential drug candidates to find one that is both safe and effective.
Model Interpretability:
AI systems face challenges related to the ability to engage in coherent conversations and provide new information, but are not ideal techniques to generate new content without human oversight.
Validation Requirements
To save money on R&D, computational studies might screen and enhance projected molecular features before costly animal and clinical testing, but experimental validation remains essential.
Regulatory Considerations
As we move further into integrating AI in drug development, FDA is committed to continuing to engage with all interested parties, sharing preliminary considerations, seeking input, and encouraging discussions on fostering the responsible use and deployment of these technologies.
Future Directions and Strategic Implications
Emerging Technologies
The concept of Digital Twins (DTs) translated to drug development and clinical trials describes virtual representations of systems of various complexities, ranging from individual cells to entire humans, and enables in silico simulations and experiments.
Generative AI has the potential to transform the field by leveraging recent developments in deep learning and customizing models for the needs of scientists, physicians and patients.
Integration with Clinical Research
The rapid advancement of generative artificial intelligence is reshaping pharmaceutical research and development (R&D), offering opportunities across drug discovery and development. GenAI enhances productivity by enabling virtual assistants which help automate routine tasks. It advances novel small-molecule drug design and drives new machine learning applications through synthetic data generation.
Investment in AI Infrastructure
As global investment in AI for drug discovery accelerates, so does the expectation of improved outcomes for drug programs. Future drivers for AI, particularly in healthcare, need to show disruption to existing business processes and tangible financial gains.
Strategic Recommendations for Stakeholders
For Pharmaceutical Companies
1. Infrastructure Investment
Companies should invest in computational infrastructure and data management systems capable of supporting generative AI workflows. FDA has seen a significant increase in the number of drug and biologic application submissions using AI/ML components, indicating industry-wide momentum.
2. Regulatory Engagement
The FDA encourages sponsors to have early engagement with the agency about AI credibility assessment or the use of AI in human and animal drug development.
3. Interdisciplinary Teams
Build teams combining expertise in medicinal chemistry, computational biology, data science, and regulatory affairs to maximize AI's potential.
For Biotech Startups
1. Focus on Validation
Ensure robust experimental validation of AI-generated predictions to build credibility with partners and regulators.
2. Leverage Collaborative Frameworks
FDA recognizes the need to learn from experts and experiences across sectors, going beyond traditional sectors into technology and ethics.
For Research Institutions
1. Foundational Research
Continue advancing generative AI methodologies specifically tailored for drug discovery applications.
2. Training Programs
The advent of novel AI applications, such as generative AI and large language models, will require increased education and coordination to enhance AI knowledge among staff members.
For Regulatory Agencies
1. Continued Framework Development
CBER, in coordination with others in FDA, developed a regulatory framework for the safe and responsible use of AI throughout the biological product lifecycle.
2. International Harmonization
CBER participated in the AI Regulatory & International Symposium, which was co-hosted by South Korea's Ministry of Food and Drug Safety and the FDA in February 2024.
Conclusion: A Transformative Era in Drug Development
Generative AI represents the most significant technological advancement in pharmaceutical R&D in decades. With over 500 submissions with AI components received by FDA from 2016 to 2023 and the establishment of the CDER AI Council in 2024, the regulatory framework is evolving to support responsible innovation.
Key Takeaways:
Economic Impact: Drug development costs of $879.3 million per approved drug can potentially be reduced through AI-assisted strategies showing cost reductions ranging from 9.9% to 27.1%.
Technical Maturity: Generative models including RNNs, VAEs, AAEs, and GANs have demonstrated capability in de novo drug design.
Regulatory Support: The FDA issued its first guidance on the use of AI for the development of drug and biological products in 2025.
Industry Momentum: 45% of clinical pharmacology professionals prioritize AI for molecule design and optimization in the next 5-10 years.
Collaborative Approach: Success requires mutual learning between FDA, industry, technology sectors, and ethics experts.
The Path Forward:
AI/ML will undoubtedly play a critical role in drug development, and FDA plans to develop and adopt a flexible risk-based regulatory framework that promotes innovation and protects patient safety.
As generative AI enhances productivity by enabling virtual assistants, advances novel small-molecule drug design, and drives new machine learning applications, organizations that strategically integrate these technologies while maintaining rigorous validation standards will lead the next era of pharmaceutical innovation.
The convergence of generative AI and drug discovery is not merely incremental improvement—it represents a fundamental reimagining of how therapeutic molecules are designed, validated, and brought to patients. The question for pharmaceutical leaders is no longer whether to adopt AI, but how quickly and effectively they can integrate these transformative capabilities into their development pipelines.
Frequently Asked Questions (FAQ)
Q1: What is generative AI in drug discovery?
Generative AI uses algorithms or models to perform tasks such as learning, making decisions, and making predictions. ML allows models to be developed by training algorithms through analysis of data, without models being explicitly programmed. In drug discovery, it creates entirely new molecular structures optimized for therapeutic properties.
Q2: How much does traditional drug development cost?
The estimated mean cost of developing a new drug is approximately $879.3 million when both costs of failures and capital are included, with costs ranging from $378.7 million for anti-infectives to $1756.2 million for pain and anesthesia drugs.
Q3: How long does drug development take?
In drug development, the clinical phase lasts an average of around 95 months compared to 31 months for the non-clinical phase, totaling over 10 years from discovery to approval.
Q4: What percentage of drug development costs come from clinical trials?
Clinical trials comprise the largest portion of overall drug development costs at $117.4 million, accounting for around 68 percent of out-of-pocket R&D expenditures.
Q5: How many AI-related submissions has the FDA received?
CDER has received over 500 submissions with AI components from 2016 to 2023, with more than 100 submissions reported in 2021 alone.
Q6: What regulatory frameworks has the FDA established for AI in drug development?
The FDA issued draft guidance in 2025 providing recommendations on the use of artificial intelligence intended to support a regulatory decision about a drug or biological product's safety, effectiveness or quality. This is the first guidance the agency has issued on the use of AI for the development of drug and biological products.
Q7: What are the main types of generative AI used in drug discovery?
The state-of-the-art generative models include recurrent neural networks (RNNs), variational autoencoders (VAEs), adversarial autoencoders (AAEs), and generative adversarial networks (GANs).
Q8: What cost reduction strategies show the most promise?
The strategies with the largest expected impact include Improvements in FDA Review Process Efficiency and Interactions (-27.1 percent), Adaptive Design (-22.8 percent), and Simplified Clinical Trial Protocols and Reduced Amendments (-22.2 percent).
Q9: Are there any AI-discovered drugs on the market?
As of 2024, there are no on-market medications that have been developed using an AI-first pipeline, though multiple candidates are in clinical trials.
Q10: How is industry responding to AI in drug discovery?
Several biopharmaceutical companies, such as Bayer, Roche, and Pfizer, have teamed up with IT companies to develop platforms for the discovery of therapies in areas such as immuno-oncology and cardiovascular diseases.
Q11: What are the main challenges in applying AI to drug discovery?
One of the biggest challenges is screening the vast number of potential drug candidates to find one that is both safe and effective. Additionally, AI-generated content requires major human edits and corrections, highlighting the need for human expertise.
Q12: What is the clinical pharmacology community's view on AI priorities?
45% of professionals prioritize AI's application in molecule design and optimization, followed by clinical trials and development (28%), target discovery and validation (20%), and preclinical testing and screening (7%).
References
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. (2024). "Artificial Intelligence for Drug Development."
U.S. Food and Drug Administration. (2025). "FDA Proposes Framework to Advance Credibility of AI Models Used for Drug and Biological Product Submissions." Press Release, January 7, 2025.
U.S. Food and Drug Administration. (2024). "Using Artificial Intelligence & Machine Learning in the Development of Drug and Biological Products: Draft Guidance."
Sertkaya, A., Beleche, T., Jessup, A., & Sommers, B. D. (2024). "Costs of Drug Development and Research and Development Intensity in the US, 2000-2018." JAMA Network Open, 7(6), e2415445.
U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation (ASPE). (2024). "Drug Development: Evaluating the Potential Impacts of Different Clinical Trial Strategies."
U.S. Department of Health and Human Services, ASPE. (2024). "Examination of Clinical Trial Costs and Barriers for Drug Development."
U.S. Food and Drug Administration. (2024). "Q&A with FDA: AI in Clinical Trial Design and Research."
Gangwal, A., Ansari, A., Ahmad, I., Azad, A. K., Kumarasamy, V., Subramaniyan, V., & Wong, L. S. (2024). "Unleashing the power of generative AI in drug discovery." Drug Discovery Today, 29(6), 103992. PubMed PMID: 38663579.
Chen, Y., et al. (2024). "Generative AI in drug discovery and development: the next revolution of drug discovery and development would be directed by generative AI." International Journal of Surgery, PMC11444559.

