How Pharma is Monetizing Biomarker Insights


The pharmaceutical industry stands at a pivotal intersection where precision medicine meets commercial opportunity. Biomarkers measurable biological indicators that signal disease states or treatment responses have evolved from research tools into strategic assets driving revenue growth and market differentiation. This transformation is reshaping how pharmaceutical companies develop drugs, segment markets, and capture value in an increasingly competitive landscape.
The Biomarker Revolution: By the Numbers
The biomarker-driven precision medicine approach has fundamentally altered pharmaceutical development. According to the FDA's Biomarker Qualification Program, 80 biomarker qualification initiatives were launched through February 2025, with academic organizations representing 70% of applicants, followed by pharmaceutical-related industries at 55%.
Growth Trajectory of Precision Medicine Initiatives
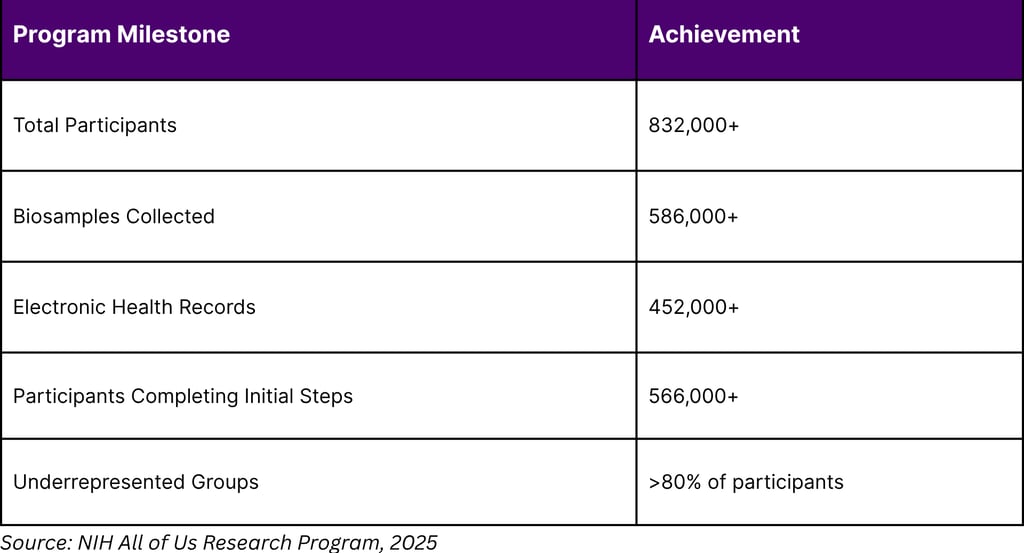
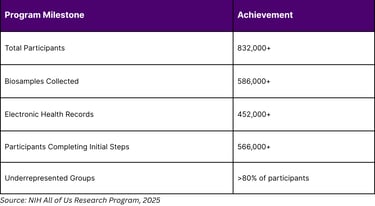
The NIH's All of Us Research Program, launched in May 2018, exemplifies the scale of biomarker-driven research. As of 2025, the program has enrolled over 832,000 participants, collected more than 586,000 biosamples, and aggregated 452,000 electronic health records. This massive dataset with over 80% of participants from historically underrepresented groups provides unprecedented opportunities for biomarker discovery and validation.
The Companion Diagnostics Market: A $170 Billion Opportunity
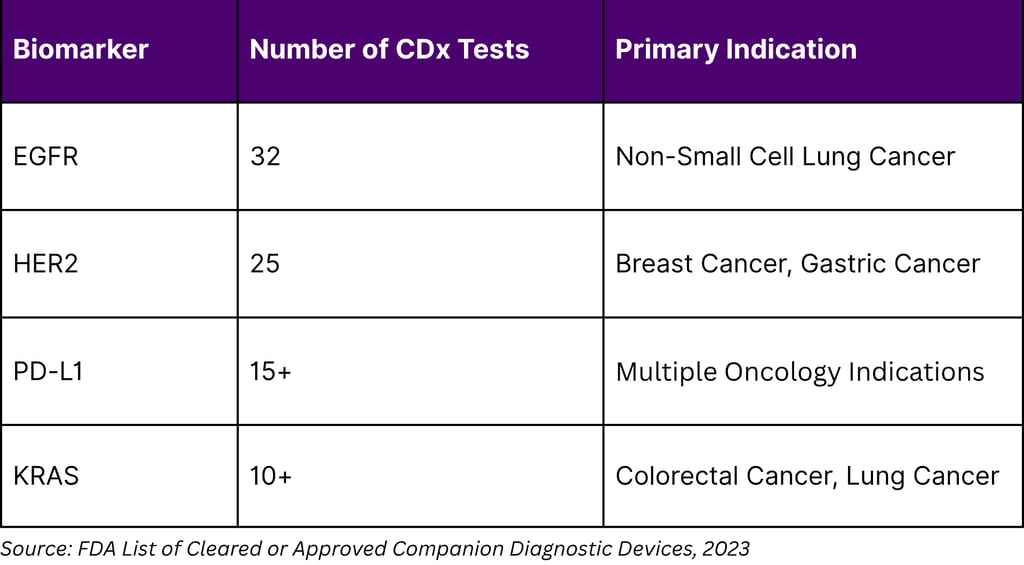
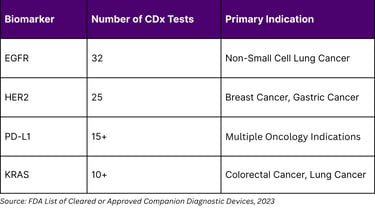
Companion diagnostics (CDx) tests that identify patients most likely to benefit from specific therapies represent a critical monetization pathway for biomarker insights. As of August 2023, the FDA had approved 56 companion diagnostic assays linked to 65 drugs and drug combinations. However, this represents only 34 unique biomarkers, indicating significant concentration in the market.
Evolution of FDA-Approved Companion Diagnostics
The companion diagnostics landscape has experienced exponential growth since the first CDx approval in 1998:
1998-2010: Modest growth, dominated by immunohistochemistry (IHC) and in situ hybridization (ISH)
2010-2023: Significant acceleration with PCR and next-generation sequencing (NGS) assays
2023: 56 total CDx assays approved, supporting precision medicine across multiple indications
Most Frequently Targeted Biomarkers
The Orphan Drug-Biomarker Nexus
The Orphan Drug Act of 1983 has created a lucrative framework for biomarker-driven drug development in rare diseases. Between 1983 and 2022, the FDA granted 6,340 orphan drug designations representing 1,079 rare diseases. Notably, 16% of orphan-designated drugs between 2009-2015 were based on predictive biomarkers that segmented diseases into rarer subsets.
Orphan Drug Designation Trends
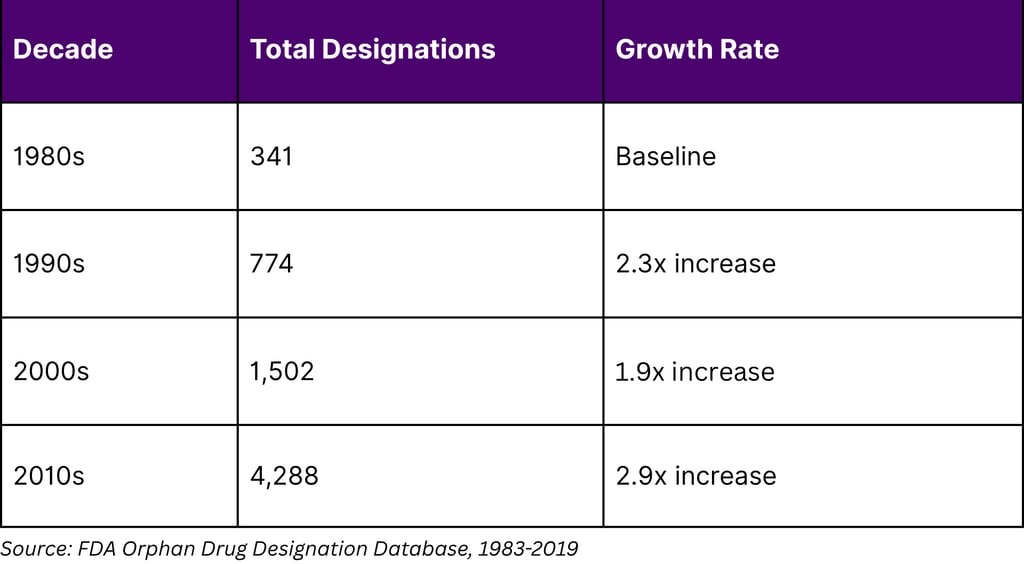
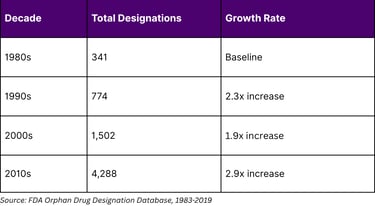
In 2022, orphan drugs represented 49% of all novel drugs and biologics approved by the FDA, demonstrating the commercial viability of precision medicine approaches in rare diseases. The financial incentives include 25% tax credits on clinical trial costs, exemption from Prescription Drug User Fee Act fees (valued at nearly $3 million), and potential 7-year market exclusivity.
Five Strategic Revenue Models for Biomarker Monetization
1. Market Segmentation Through Biomarker-Defined Indications
Pharmaceutical companies increasingly use biomarkers to carve out exclusive market segments. For example, non-small cell lung cancer (NSCLC) affects approximately 250,000 Americans annually. By identifying EGFR mutation-positive patients (40-80% of NSCLC cases), companies create targeted markets of 75,000-150,000 patients small enough for orphan designation yet large enough for substantial revenues.
2. Premium Pricing for Precision Therapies
Biomarker-guided therapies command premium pricing due to demonstrated efficacy in selected populations. The median annual cost for an orphan drug in 2016 exceeded $32,000, with the top ten therapies averaging $14,909 for the most widely used medications. This pricing reflects the value proposition of treating only responders, reducing healthcare costs associated with ineffective treatments.
3. Lifecycle Extension Through Indication Expansion
Of the 3,269 unique products receiving orphan drug designation between 1983-2019, 25% received multiple designations. Over 60% of products with 10 or more designations were antineoplastics or immunomodulators, revealing a "repositioning continuum" strategy where companies leverage biomarkers to identify new patient populations for existing compounds.
4. Regulatory Acceleration and De-Risking
The FDA's Biomarker Qualification Program provides a pathway to accelerate drug development. Qualified biomarkers can be used across multiple drug development programs without requiring sponsors to reconfirm validity. This public-private partnership approach reduces development costs and timelines while increasing probability of regulatory success.
5. Data Monetization Through Research Collaborations
The TOPMed (Trans-Omics for Precision Medicine) program has sequenced nearly 200,000 genomes from diverse populations, creating valuable datasets for biomarker discovery. The second phase is generating genomic, epigenomic, transcriptomic, proteomic, and metabolomic data multi-omics approaches that enable pharmaceutical companies to identify novel therapeutic targets and stratification strategies.
The Clinical Trial Transformation
Approximately 100 drugs in Phase II, III, and IV clinical trials listed diagnostic information as part of primary/secondary outcomes or inclusion/exclusion criteria on ClinicalTrials.gov. However, only nine examples met the strict FDA definition of companion diagnostic with specific diagnostics included in trial protocols. This gap represents both a challenge and opportunity for pharmaceutical developers.
Success Rates: Biomarker-Enriched vs. Traditional Trials
Studies analyzing biomarker use in drug interventional clinical trials revealed that 58.5% of biomarker-utilizing studies were sponsored by the United States, with studies conducted mainly in the sponsor's home region (80.3%). Oncology represented 37.1% of biomarker applications, reflecting the therapeutic area's leadership in precision medicine adoption.
Implementation Framework: From Discovery to Deals
Stage 1: Biomarker Discovery and Validation (Years 1-3)
Investment: $5-15 million
Activities: Genomic screening, assay development, retrospective validation
Milestone: Letter of Intent submission to FDA
Stage 2: Clinical Qualification (Years 3-6)
Investment: $20-50 million
Activities: Prospective clinical trials, Qualification Plan submission
Milestone: FDA acceptance of biomarker context of use
Stage 3: Regulatory Approval and Commercialization (Years 6-10)
Investment: $50-200 million (combined drug-diagnostic)
Activities: Pivotal trials, Full Qualification Package submission
Milestone: Simultaneous drug and CDx approval
Stage 4: Market Expansion and Lifecycle Management (Years 10+)
Investment: $10-30 million per new indication
Activities: Indication expansion, real-world evidence generation
Milestone: Multiple approved indications across biomarker-defined populations
Overcoming Key Challenges
Challenge 1: Limited Biomarker Diversity
Despite 170 FDA-approved CDx tests, only 34 unique biomarkers have been qualified. This concentration suggests untapped opportunities in novel biomarker development beyond established targets like EGFR, HER2, and PD-L1.
Challenge 2: Assay Standardization
The transition from research-grade biomarker tests to clinical-grade companion diagnostics requires rigorous analytical validation. CLIA-certified laboratories must demonstrate reproducibility, sensitivity, and specificity across diverse patient populations.
Challenge 3: Health Equity and Access
While the All of Us Research Program prioritizes diversity, historical genomic datasets have overrepresented individuals of European descent. Ensuring biomarker validity across ancestries is essential for both ethical and commercial success.
Future Outlook: The Next Decade of Biomarker Commercialization
Emerging Trends
Multi-Omics Integration: The convergence of genomics, proteomics, metabolomics, and digital health data will enable more sophisticated patient stratification strategies.
Liquid Biopsy Expansion: Non-invasive biomarker detection through blood-based tests will accelerate adoption and enable real-time treatment monitoring.
AI-Powered Biomarker Discovery: Machine learning algorithms analyzing massive datasets like All of Us will identify novel biomarker signatures with unprecedented speed.
Tissue-Agnostic Therapies: FDA guidance on tissue-agnostic drug development enables treatments targeting specific molecular alterations across multiple cancer types, expanding addressable markets.
Market Projections
Based on current trajectories:
Orphan drug designations will continue growing at 2.5-3x per decade
Companion diagnostics will expand beyond oncology into metabolic, neurological, and infectious diseases
Biomarker qualification submissions will increase as industry gains experience with FDA pathways
Conclusion
The biomarker-driven precision medicine revolution represents a fundamental shift in pharmaceutical business models. Companies that successfully navigate the complex pathway from biomarker discovery through regulatory qualification to commercial launch will capture significant value in an increasingly competitive market. The convergence of massive datasets, advanced analytics, supportive regulatory frameworks, and financial incentives creates unprecedented opportunities for those who can translate biological insights into clinical and commercial success.
The question is no longer whether to invest in biomarker-driven drug development, but how quickly companies can scale these capabilities to remain competitive in the precision medicine era.
Frequently Asked Questions
Q: What is the difference between a biomarker and a companion diagnostic?
A: A biomarker is any measurable biological characteristic that indicates disease state or treatment response. A companion diagnostic is a medical device (often a test) that identifies the presence or level of a specific biomarker to guide therapeutic decision-making for a corresponding drug.
Q: How long does FDA biomarker qualification typically take?
A: The FDA biomarker qualification process involves three stages: Letter of Intent (LOI), Qualification Plan (QP), and Full Qualification Package (FQP). The complete process typically spans 3-7 years, depending on the complexity of the biomarker and the quality of supporting evidence.
Q: Can a drug be approved without a companion diagnostic?
A: Yes. Biomarkers do not need to be formally qualified to be used in drug development. Many drugs are approved with biomarker information in their labels without a qualified companion diagnostic. However, qualified biomarkers provide regulatory certainty and can be used across multiple drug development programs.
Q: What percentage of rare diseases have FDA-approved treatments?
A: Based on FDA data, approximately 5% of rare diseases have an FDA-approved drug, and up to 15% have at least one drug that has been developed and shown promise in treatment, diagnosis, or prevention.
Q: How do orphan drug incentives support biomarker-driven development?
A: The Orphan Drug Act provides 25% tax credits for clinical trial costs, exemption from prescription drug user fees (valued at ~$3 million), and potential 7-year market exclusivity. These incentives make development economically viable for small patient populations defined by biomarkers.
Q: What is the success rate for orphan drug approvals?
A: Of the 3,269 unique products receiving orphan drug designation between 1983-2019, only 508 (16%) received approval for an orphan-designated indication. However, approval rates vary significantly by therapeutic area, ranging from 0-30%.
Q: How are pharmaceutical companies using the All of Us Research Program data?
A: The All of Us Researcher Workbench provides approved researchers access to electronic health records, genomic data, survey responses, and biospecimen data from 832,000+ participants. This enables biomarker discovery, validation of therapeutic targets, and identification of patient subgroups for clinical trials.
Q: What therapeutic areas show the most biomarker-driven development activity?
A: Oncology leads with 37.1% of biomarker-utilizing clinical studies and 1,910 (37%) of orphan drug designations. Neurology follows with 13% of orphan designations, and infectious diseases represents 9%.
References
All of Us Research Program Investigators. (2019). The “All of Us” Research Program. New England Journal of Medicine, 381(7), 668–676.
Collins, F. S., & Varmus, H. (2015). A new initiative on precision medicine. New England Journal of Medicine, 372(9), 793–795.
FDA. (2016). BEST (Biomarkers, EndpointS, and Other Tools) Resource. U.S. Food and Drug Administration & National Institutes of Health.
FDA. (2021). Designating an orphan product: Drugs and biological products. U.S. Food and Drug Administration.
FDA. (2022). Focus area: Biomarkers. U.S. Food and Drug Administration.
FDA. (2023). About biomarkers and qualification. U.S. Food and Drug Administration.
FDA. (2023). Rare diseases at FDA. U.S. Food and Drug Administration.
FDA. (2023). Status of biomarker qualification submissions. U.S. Food and Drug Administration.
FDA. (2024). Guidance for industry: Rare diseases—Considerations for the development of drugs. U.S. Food and Drug Administration.
Fradkin, J. E., Hanlon, M. C., & Rodgers, G. P. (2016). NIH Precision Medicine Initiative: Implications for diabetes research. Diabetes Care, 39(7), 1080–1084.
Lanthier, M., Miller, K. L., Nardinelli, C., & Woodcock, J. (2023). A comprehensive study of rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act. Orphanet Journal of Rare Diseases, 18(1), 163. (Data sourced from FDA internal database)
Miller, K. L., Fermaglich, L. J., & Maynard, J. (2021). Using four decades of FDA orphan drug designations to describe trends in rare disease drug development. Orphanet Journal of Rare Diseases, 16(1), 265. (Data sourced from FDA Orphan Drug Designation Database)
National Institutes of Health. (2025). Precision medicine activities. National Heart, Lung, and Blood Institute.
NIH. (2025). All of Us Research Program. National Institutes of Health.
Shahzad, M., Upshaw, J. N., Schafer, P. H., & Stern, A. D. (2025). Participants in the FDA’s Biomarker Qualification Program. Clinical Pharmacology & Therapeutics. (Data sourced from FDA Biomarker Qualification Program)

