How South Korea Is Quietly Rewiring Global Drug Development


While the pharmaceutical world focuses on established powerhouses like the United States, Switzerland, and Germany, a new contender is rapidly ascending the global hierarchy. South Korea is systematically transforming itself from a regional player into a formidable force in biopharmaceutical innovation, contract manufacturing, and clinical research. With aggressive government backing, world-class infrastructure, and strategic positioning in the biologics market, the Korean pharmaceutical sector is not merely growing—it's being architected to dominate.
The Government Blueprint: A Strategic National Vision
What distinguishes South Korea from other emerging pharmaceutical markets is the precision and ambition of government intervention. In March 2023, the Korean government announced the 3rd Master Plan for Fostering and Supporting Pharmaceutical and Bio Industries (2023-2027) with a vision to "emerge as a global leader of pharmaceutical and bio industries."
This comprehensive strategy outlines specific, measurable goals that demonstrate Korea's commitment to becoming a pharmaceutical superpower.
K-Bio Strategic Targets (2023-2030)
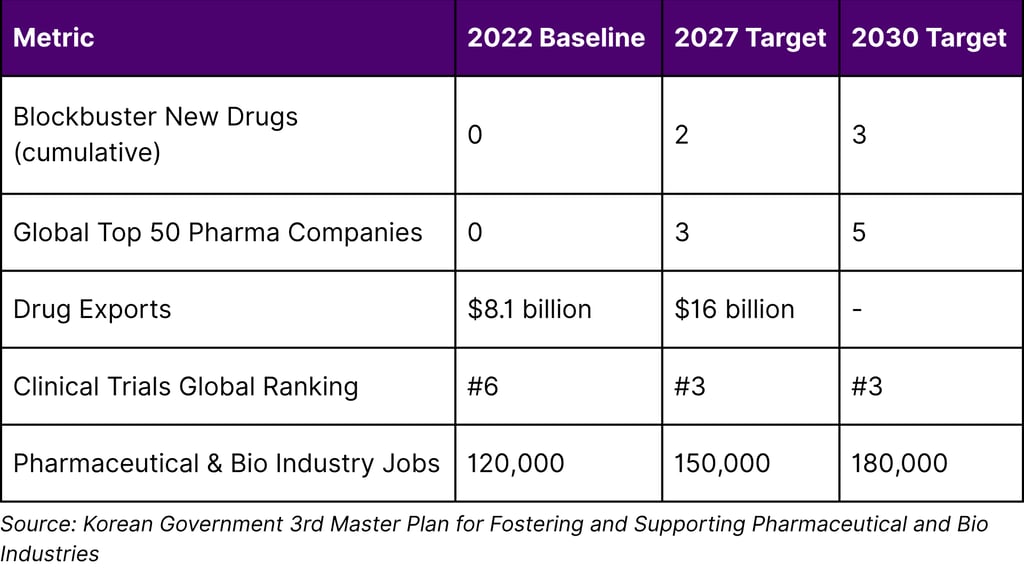
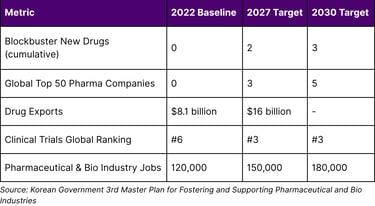
The plan's ambition is clear: double drug exports within five years, develop multiple blockbuster drugs, and position Korea as the third-largest clinical trial hub globally.
The Numbers Tell a Compelling Story
According to the Ministry of Food and Drug Safety's official statistics, Korea's pharmaceutical market exceeded 25 trillion won (approximately $21 billion USD) in 2021, growing at an average annual rate of 4% over five years. This growth accelerated with increased production of COVID-19 vaccines and treatments.
Korea's Pharmaceutical Industry Performance (2017-2021)
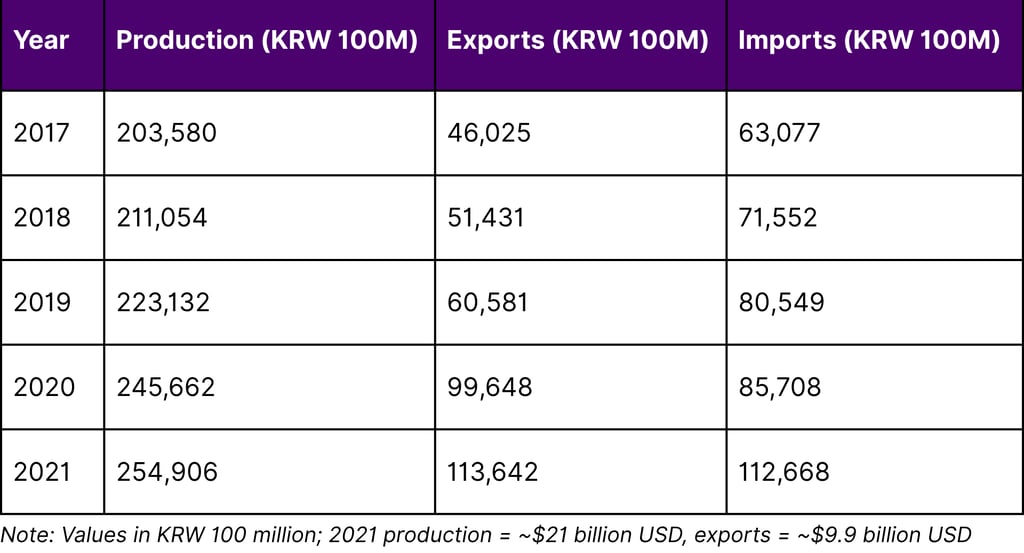
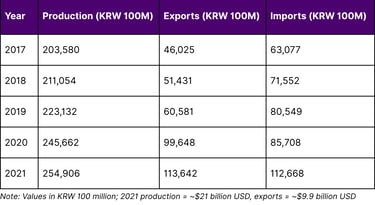
Domestic pharmaceutical production reached 25.49 trillion won in 2021, up 3.8% year-on-year, with an average annual growth rate of 5.8% over five years. More impressively, pharmaceutical exports reached 11.36 trillion won (about $9.9 billion USD) in 2021, up 14.0% year-on-year.
Four Strategic Pillars of Growth
The government's master plan focuses on four comprehensive areas designed to transform Korea into a global pharmaceutical leader:
1. Strengthening R&D Capabilities
The government is prioritizing strategic R&D investments to create global blockbuster drugs, respond to health security needs, and promote digital transformation of drug development through AI and big data integration.
2. Expanding Export Support
Korea is increasing investments in pharmaceutical and bio industries while strengthening financial support for startups and industrializing core exports in the pharmaceutical and bio sectors.
3. Developing Specialized Workforce
The government plans to train 110,000 people into production and research personnel, operate the WHO-designated Global Training Hub for Biomanufacturing, and create a comprehensive ecosystem for training pharmaceutical and bio professionals.
4. Upgrading Systems and Infrastructure
This includes innovating regulations to meet global standards, providing strategic support for clinical trials, and strengthening infrastructure across the pharmaceutical value chain.
World-Class Infrastructure: 18 Bio Clusters and Growing
Korea has established eighteen specialized bio clusters operating at full scale across the country, each with distinct specializations:
Major Bio Clusters in South Korea:
Seoul Hongneung Biomedical R&D Cluster - Research and development hub
Incheon Songdo Bio Cluster - Biopharmaceuticals and CMO specialization
Osong High-tech Medical Complex (Chungbuk) - Biopharmaceuticals and BT-based medical devices
Gyeonggi Gwanggyo Techno Valley - Healthcare innovation
Daegu Gyeongbuk High-tech Medical Complex - Integrated biomedical development
Andong Vaccine Industry Cluster - Vaccine production and research
Source: InvestKOREA (Korea Trade-Investment Promotion Agency), 3rd Master Plan
The government plans to add two additional national industrial complex candidate sites:
Andong, Gyeongbuk (Pungsan-eup): 1.32 million ㎡ focusing on bio and medical (vaccines, HEMPs)
Gangneung, Gangwon (Gujeong-myeon): 930,000 ㎡ focusing on natural products and bio (food, energy)
Manufacturing Excellence: The CDMO Advantage
South Korea has methodically constructed what may become the world's largest biologics manufacturing capacity. According to government records, Samsung Biologics produced 424,000 liters in 2022, boasting the world's biggest contract production capacity, with plans to add 360,000 liters by 2026.
Lotte Biologics has built three biopharmaceutical contract production plants with a capacity of 120,000 liters in Korea, with plans to increase capacity to 360,000 liters by 2027 in Korea alone. The company also acquired Bristol-Myers Squibb's bio plant in Syracuse, New York in May 2022.
South Korea's Biomanufacturing Capacity Growth
Samsung Biologics:
2022: 424,000 liters (world's largest)
2026 Target: 784,000 liters total
Lotte Biologics:
Current: 120,000 liters (Korea)
2027 Target: 360,000 liters (Korea)
Plus: Syracuse, NY facility (USA)
Source: InvestKOREA, Government Press Releases
Clinical Trial Excellence: Seoul's Global Leadership
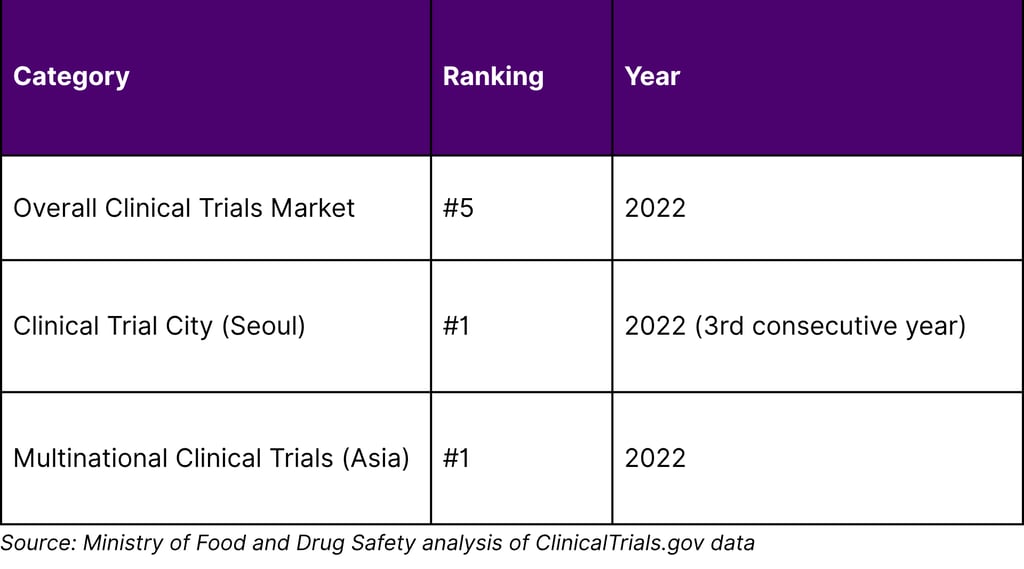
Korea's clinical trial infrastructure has earned international recognition. According to Ministry of Food and Drug Safety data, Korea's share in the industry-led clinical trial market ranked 5th globally in 2022, up from 6th in 2021. Most notably, Seoul has ranked 1st in global clinical trial city rankings for three consecutive years.
Korea's Clinical Trial Competitive Position
International Recognition and Standards
Korea has achieved critical international certifications:
2014: Joined Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S)
2016: Joined International Council for Harmonisation (ICH)
May 2019: Became the 7th country listed on EU Whitelist (exempt from GMP written confirmation)
February 2022: Solely designated as WHO Global Training Hub for Biomanufacturing (GTH-B)
Biosimilar Leadership
Korea has established particular expertise in biosimilars. As of December 2022, nine out of forty biosimilar products approved by the United States Food and Drug Administration were developed by Korea—the second-highest number following the United States.
This biosimilar expertise serves as both a revenue generator and a technical foundation for developing novel biologics, positioning Korea as a critical player in the global biologics supply chain.
Global Partnerships: R&D Collaboration Ecosystem
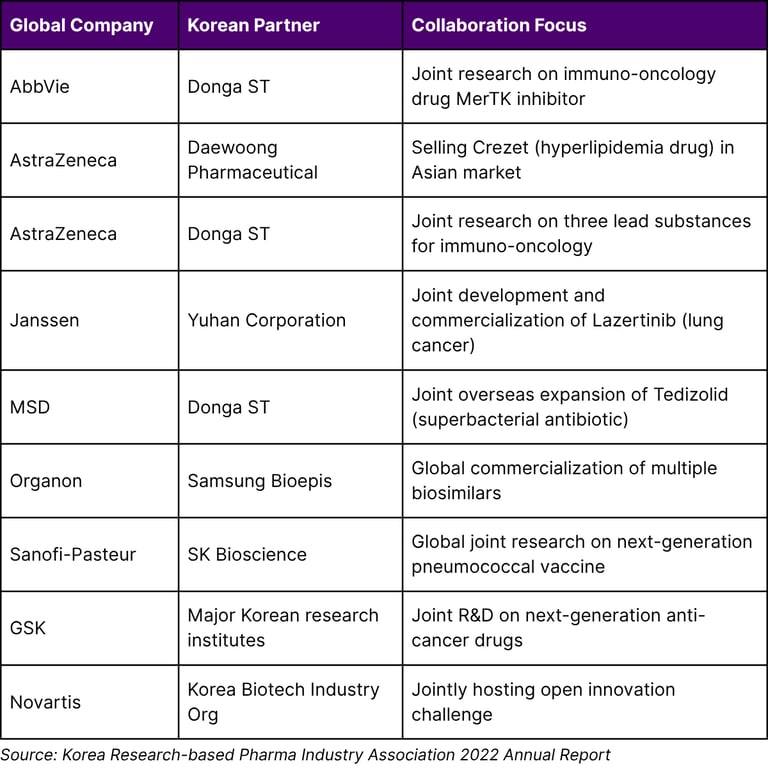
According to the Korea Research-based Pharma Industry Association, 31 global pharmaceutical companies operating in Korea invested approximately 715.3 billion won (about $600 million) in R&D costs in 2021, conducting 1,590 clinical studies in Korea in that year alone.
Major International Pharmaceutical Collaborations
Government Investment: Putting Money Behind the Vision
The Ministry of Health and Welfare's 2023 Action Plan outlined significant financial commitments:
Strategic R&D Investments:
Pharmaceutical R&D: 25 trillion won through 2027
Medical devices R&D: 10 trillion won through 2027
K-Bio Vaccine Fund: 1 trillion won total (established 2023-2025)
Technology Development Programs:
Essential vaccines localization: 215.1 billion won through 2029
mRNA vaccines: 21 billion won through 2023
Antivirus medicines: 46.4 billion won through 2029
Artificial blood technology: 47.1 billion won through 2027 (joint ministry project)
Xenotransplants: 38 billion won through 2027
Source: Ministry of Health and Welfare 2023 Action Plan
Digital Transformation and Advanced Technology
The government is actively promoting digital transformation of drug development, including AI and big data applications. Specific initiatives include:
Health Information Highway System: Enabling data connectivity across healthcare institutions
Clinical and Genomic Databank: Building a one million-person database
Big Data for Major Diseases: Utilizing big data to overcome cancer and other major diseases
Smart Healthcare Infrastructure: Supporting customization of smart features in public and private hospitals
Regulatory Innovation: Creating a Favorable Environment
Korea is implementing continuous regulation reform to accelerate innovation:
Comprehensive screening system for innovative medical devices
Extended deferment of new medical technology assessments
Cross-ministry governance to support all production stages from basic R&D to market entry
Entry of new products before assessment for breakthrough technologies
Foreign Investment Success Stories
The COVID-19 pandemic triggered increased production and R&D investments in vaccines and biopharmaceuticals from global players:
Cytiva (Danaher, USA): Invested in Songdo, Incheon to establish a disposable culture bag production facility for vaccine production processes.
Sartorius (Germany): Building a production facility in Songdo, Incheon to produce biopharmaceuticals and raw materials for biotechnology R&D in Korea.
Prestige Biopharma (Singapore): Continued expansion of vaccine and biopharmaceutical CDMO facility in Osong, Chungcheongbuk-do, and established Prestige Biopharma Korea Innovation Discovery Center for advanced biopharmaceutical R&D and global cooperation.
Source: InvestKOREA Foreign Investment Success Stories
Strategic Focus on Blockbuster Drug Development
The government's 2023 action plan specifically targets developing "two blockbuster-level new drugs" by 2027, with Korea aiming to be the world's fifth-ranked exporter of medical devices by the same year.
To achieve this, Korea is:
Expanding public-private investment for pharmaceutical R&D (25 trillion won through 2027)
Starting K-Bio Vaccine Fund investments (1 trillion won through 2025)
Actively responding to regulatory tightening in major countries
Providing strategic support by industry and region to develop new markets
The WHO Designation: Global Capacity Building Hub
In February 2022, Korea received a prestigious international designation when the World Health Organization named it the sole Global Training Hub for Biomanufacturing (GTH-B). This hub is designed to help develop bio capabilities of low and middle-income countries having difficulties procuring vaccines, positioning Korea as a champion in resolving global inequality in vaccine supply and building the global healthcare safety net.
This designation reflects Korea's growing international stature and positions it as a capacity-building center for emerging pharmaceutical markets worldwide.
Challenges and Strategic Responses
Despite impressive momentum, Korea faces structural challenges that the government is actively addressing:
Domestic Market Size: While growing steadily at 4-5% annually, the domestic market remains smaller than major pharmaceutical powers, necessitating global commercialization strategies.
Supply Chain Dependencies: Korea remains dependent on imported raw materials and specialized equipment. The government is investing in domestic supply chain development as part of the master plan.
Talent Development: Meeting the target of 150,000 jobs by 2027 (from 120,000 in 2021) requires sustained investment in education and training programs, which the government has prioritized through the 110,000-person workforce development initiative.
Investment Implications for Global Stakeholders
For pharmaceutical companies, contract research organizations, and life sciences investors, Korea's trajectory carries several strategic implications:
CDMO Capacity: Korea's massive biologics manufacturing capacity makes it an essential partner for global biologics development and commercialization.
Clinical Trial Infrastructure: Seoul's #1 global ranking and Korea's #5 position offer efficient pathways for Asian market studies with international regulatory standards.
Innovation Ecosystem: The emerging R&D ecosystem presents partnership and licensing opportunities, particularly in biosimilars and biologics.
Government Support: The comprehensive, well-funded government support creates a stable, predictable environment for long-term investments.
The 2030 Vision: From Aspiration to Reality
The Korean government has established a clear roadmap with specific milestones:
By 2027:
2 blockbuster drugs created
$16 billion in pharmaceutical exports (doubled from 2022)
3 companies in global top 50
#3 global ranking in clinical trials
150,000 industry jobs
By 2030:
3 blockbuster drugs (cumulative)
5 companies in global top 50
180,000 industry jobs
Sustained position as global pharmaceutical leader
Given the government's track record of execution, substantial financial commitments, and current progress trajectories, these targets appear increasingly achievable.
Conclusion: A Systematic Rise to Global Prominence
South Korea's pharmaceutical ascent is not an accident or market fluke—it is the result of deliberate, systematic government planning combined with private sector execution capability. The combination of the 3rd Master Plan's clear targets, substantial financial investments ($1 trillion vaccine fund, 25 trillion won pharma R&D), world-class infrastructure (18+ bio clusters, 784,000+ liter biomanufacturing capacity), international regulatory recognition (PIC/S, ICH, EU Whitelist, WHO GTH-B designation), and strategic workforce development positions Korea uniquely in the global pharmaceutical landscape.
For global pharmaceutical stakeholders, Korea is transitioning from an optional market to a strategic necessity. The country's integration into global drug development value chains will only deepen, making Korean partnerships, facilities, and capabilities increasingly central to pharmaceutical innovation and manufacturing worldwide.
The question is no longer whether Korea will become a major pharmaceutical power—it's how quickly the country will dominate specific segments and which global players will position themselves early to benefit from Korea's rise as the next pharmaceutical superpower.
Frequently Asked Questions (FAQs)
Q1: What makes South Korea's pharmaceutical strategy unique compared to other countries?
South Korea's approach is distinguished by comprehensive government planning with specific numerical targets, substantial financial commitments (1 trillion won vaccine fund, 25 trillion won pharma R&D through 2027), world-class biomanufacturing infrastructure (Samsung Biologics' 784,000L capacity), international regulatory recognition (PIC/S, ICH, EU Whitelist, WHO Global Training Hub), and coordinated cross-ministry support. Unlike many countries with general support programs, Korea has a detailed roadmap with measurable goals targeting Global Top 5 status by 2030.
Q2: What are the specific targets of Korea's K-Bio 2030 plan?
The 3rd Master Plan (2023-2027) targets: doubling drug exports from $8.1 billion (2022) to $16 billion (2027), creating 2 blockbuster drugs by 2027 and 3 by 2030, developing 3-5 companies in the Global Top 50 pharmaceutical firms, achieving #3 global ranking in clinical trials (from #6 in 2021), and creating 30,000-60,000 new jobs (150,000 by 2027, 180,000 by 2030).
Q3: How has Korea's pharmaceutical production and export performance been?
According to Ministry of Food and Drug Safety data, pharmaceutical production reached 25.49 trillion won ($9.9 billion) in 2021, up 14.0% year-on-year, with particularly strong growth in 2020 (64.5% increase) driven by COVID-19 vaccines and treatments.
Q4: What is Korea's position in global clinical trials?
Korea ranked #5 globally in industry-led clinical trials in 2022 (up from #6 in 2021). Seoul specifically ranked #1 in global clinical trial city rankings for three consecutive years. Korea also ranks #1 among Asian countries for multinational clinical trials. The government targets reaching #3 globally by 2027.
Q5: How many bio clusters does Korea have and what do they specialize in?
Korea operates 18 specialized bio clusters nationwide, including: Songdo Bio Complex (biopharmaceuticals and CMO), Osong Medical Industrial Complex (biopharmaceuticals and BT medical devices), Andong Vaccine Industry Cluster (vaccine production), Daegu Gyeongbuk High-tech Medical Complex (integrated biomedical), and others across Seoul, Gyeonggi, Chuncheon, Daejeon, Gwangju, Pohang, and Jeju. The government plans to add 2 more clusters in Andong and Gangneung.
Q6: What international recognitions has Korea received?
Korea joined PIC/S in 2014 and ICH in 2016, making it globally recognized for pharmaceutical regulation. In May 2019, Korea became the 7th country on the EU Whitelist (exempt from GMP written confirmation). In February 2022, WHO designated Korea as the sole Global Training Hub for Biomanufacturing (GTH-B) to help low and middle-income countries develop bio capabilities.
Q7: How much is the Korean government investing in pharmaceutical and bio industries?
Major investments include: K-Bio Vaccine Fund of 1 trillion won (2023-2025), pharmaceutical R&D investment of 25 trillion won through 2027, medical devices R&D of 10 trillion won through 2027, essential vaccines localization of 215.1 billion won through 2029, mRNA vaccines 21 billion won through 2023, and antivirus medicines 46.4 billion won through 2029.
Q8: What is Korea's strength in biosimilars?
As of December 2022, nine out of forty biosimilar products approved by the US FDA were developed by Korea—the second-highest number globally after the United States. This expertise in biosimilars provides both revenue generation and a technical foundation for developing novel biologics, positioning Korea as critical to global biologics supply chains.
Q9: Which global pharmaceutical companies are collaborating with Korea?
Major collaborations include: AbbVie-Donga ST (immuno-oncology), AstraZeneca-Daewoong (hyperlipidemia drug for Asia), Janssen-Yuhan (lung cancer treatment), MSD-Donga ST (superbacterial antibiotic), Organon-Samsung Bioepis (biosimilars), Sanofi-Pasteur-SK Bioscience (next-gen vaccine), GSK-Korean research institutes (anti-cancer drugs), and Novartis-Korea Biotech Industry Organization (open innovation). In 2021, 31 global companies invested 715.3 billion won ($600M) in R&D in Korea.
Q10: How can international companies invest or partner in Korea's pharmaceutical sector?
InvestKOREA (Korea Trade-Investment Promotion Agency) facilitates foreign investment and partnerships. Opportunities include CDMO relationships for biologics manufacturing, clinical trial collaborations, technology licensing with Korean biotech companies, joint R&D ventures, and distribution partnerships. The government offers financial support, regulatory assistance, and access to 18 specialized bio clusters with advanced infrastructure.
References
Korean Government. (March 2023). "3rd Master Plan for Fostering and Supporting Pharmaceutical and Bio Industries (2023-2027)." Joint announcement by government ministries.
Ministry of Food and Drug Safety, Republic of Korea. (August 2022). "2021 Domestic Pharmaceutical Market and Production Records." Official government statistics.
Ministry of Health and Welfare, Republic of Korea. (January 2023). "MOHW - Action Plan for 2023." Official government press release.
InvestKOREA (Korea Trade-Investment Promotion Agency). (2024). "Pharma & Bio Industry Overview." Official government investment portal.
Ministry of Food and Drug Safety. (2023). "Analysis of Clinical Trials Registration System (ClinicalTrials.gov)." Official government data analysis.
Korea Research-based Pharma Industry Association. (2022). "Annual Report on Multinational Pharmaceutical Companies in Korea." Industry association annual report.
Korean Government Ministry of Trade, Industry and Energy. (March 2023). "Fifteen National High-tech Industrial Complexes for Creation of High-tech Industry Ecosystem." Official press release.
World Health Organization. (February 2022). "Korea Designated as Global Training Hub for Biomanufacturing (GTH-B)." Official WHO announcement.
Korea.net (Official Korean Government Portal). (2023). "K-Bio Pharmaceutical Industry Policy Updates."
InvestKOREA. (2023). "Foreign Investment Success Stories - Pharma & Bio Sector." Korea Trade-Investment Promotion Agency official case studies.

