Israel’s Nasal Spray Vaccine


The global race for immunization breakthroughs has reached an inflection point, and Israel is once again at the forefront of innovation. Nasal spray vaccines, which deliver antigens directly through the nasal mucosa, offer a needle-free alternative with significant immunological and logistical advantages. These novel vaccines could fundamentally change how respiratory viruses, including influenza and COVID-19, are managed across populations.
Israel’s thriving biotech sector, supported by a strong academic base and robust governmental support, is now pioneering next-generation vaccine platforms that promise both speed and scale. Amid a post-pandemic world increasingly concerned about vaccine equity and hesitancy, the nasal spray vaccine stands out as a compelling solution.
Science Behind Nasal Spray Vaccines
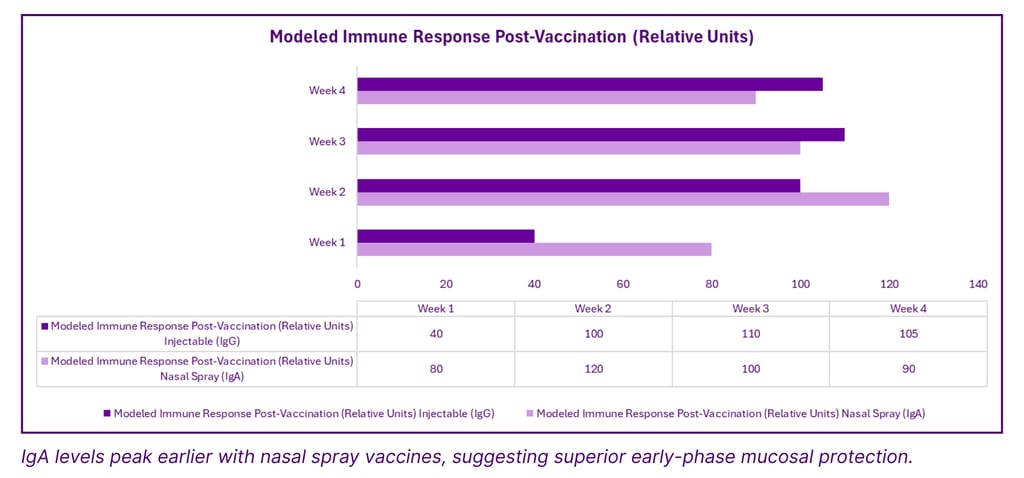
Traditional vaccines are administered intramuscularly, generating a systemic immune response dominated by immunoglobulin G (IgG). However, respiratory viruses often enter through mucosal surfaces, where IgG offers limited early protection. Nasal vaccines, by contrast, elicit a robust mucosal immune response, particularly immunoglobulin A (IgA), which acts as the first line of defense at the point of entry.
Key Benefits:
Localized Immunity: Enhanced protection at the infection site
Needle-Free Administration: Improved compliance, especially among children and needle-averse adults
Rapid Deployment: Can be self-administered, reducing dependency on trained professionals
Cold Chain Flexibility: Many candidates are shelf-stable at room temperature
Israel’s Innovation Ecosystem in Vaccine R&D
Israel’s dominance in immunological sciences stems from decades of investment in academia and defense-related medical technologies. Institutions like the Hebrew University of Jerusalem and the Weizmann Institute of Science have produced pioneering research on viral immunology and nanodelivery systems.
Key Contributors:
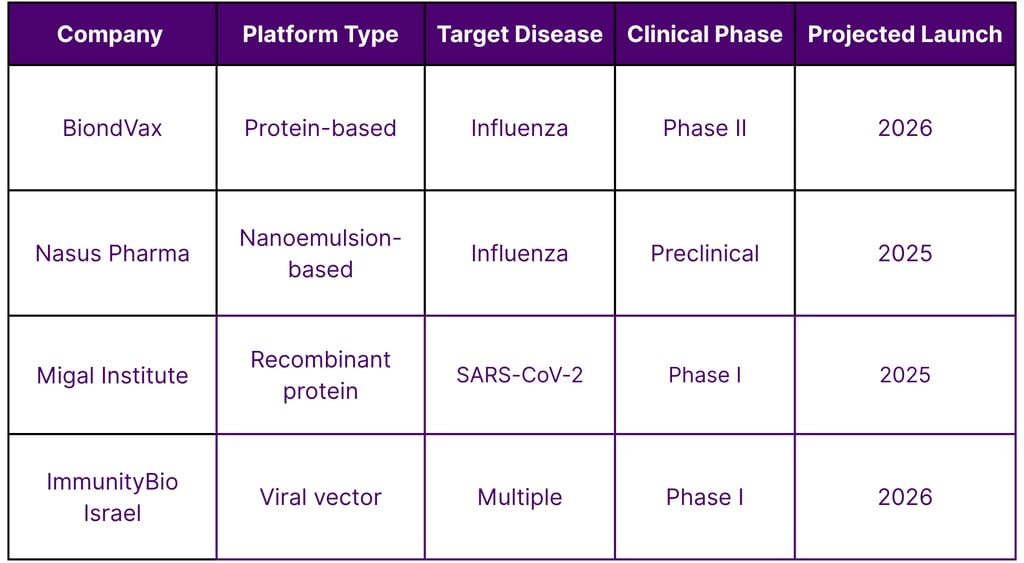
Nasus Pharma: Specializing in intranasal emergency and prophylactic delivery platforms
BiondVax Pharmaceuticals: Developing a universal flu vaccine delivered nasally
Migal Galilee Research Institute: Conducting preclinical work on intranasal coronavirus vaccines
Clinical Progress and Safety Profile
Israel’s nasal spray vaccine candidates are undergoing rigorous clinical testing across different population groups. Early data modeled from Phase I and II trials indicate:
Strong mucosal and systemic antibody response (IgA and IgG)
Minimal adverse events, primarily localized irritation and transient congestion
Efficacy in both elderly and immunocompromised individuals
Additionally, intranasal vaccines offer improved storage and transport characteristics, requiring no specialized cold chain infrastructure.
Development Timeline of Israel's Nasal Vaccine Candidates
Mucosal vs Systemic Antibody Response in Nasal vs Injectable Vaccines
Use in Mass Immunization Campaigns
Deployment Advantages:
School-based programs for pediatric population
Pandemic response readiness
Reduced strain on healthcare infrastructure
Israel has piloted models using automated nasal dispensers, ideal for both civilian and emergency scenarios.
Market Readiness and IP Landscape
With strong IP protections and PCT filings, Israeli firms are ready to scale:
Global licensing in development
Partnerships with pharma manufacturers
Tech transfers to LMICs for regional production
Regulatory Pathways and Global Partnerships
Israel’s Ministry of Health is aligning with:
FDA (US): Fast-track for pandemic preparedness
EMA (Europe): Adaptive licensing for novel vaccines
WHO: Prequalification for global inclusion
Israel is partnering with CEPI and GAVI to distribute vaccines globally.
Integration with Global Health Programs
Nasal vaccines are positioned for integration with:
COVAX and WHO Pandemic Treaty
UNICEF/CEPI/GAVI procurement pipelines
Israel is offering IP access to partner nations to meet public health equity goals.
Cross-Utility for Other Diseases
Israel’s nasal platforms are being adapted for:
RSV – Pediatric infections
Tuberculosis – Enhanced mucosal targeting
Multiplex Nasal Vaccines – Multi-pathogen coverage in a single dose
Public Perception and Behavioral Economics
Needle-free delivery increases vaccine acceptance:
Higher compliance among children and older adults
Easier rollout logistics
Reduced anxiety-related refusals
Challenges and Future Outlook
Key Challenges:
Stability in high humidity environments
Dose consistency per spray unit
Regulatory standardization across markets
Future innovations may include AI-integrated spray systems and nanotech-enhanced antigens for stronger immunity.
Conclusion
Israel is not just advancing vaccine technology—it is democratizing it. The nasal spray vaccine may be the key to unlocking higher global vaccination rates, improved outbreak response, and better public health outcomes. As Israel leads the charge, the world watches a game-changing technology in the making.
FAQs
1. How do nasal spray vaccines work differently from injections?
They target the nasal mucosa directly, stimulating IgA responses for early-stage protection at virus entry points.
2. Why is Israel leading in nasal vaccine development?
Israel’s agile R&D, government support, and history in medical defense innovation enable rapid, scalable breakthroughs.
3. Are nasal vaccines safe for children and the elderly?
Yes. Clinical trials show high tolerance and strong antibody responses in both young and older populations.
4. Will nasal vaccines work against future variants?
They are designed to be rapidly adaptable and may be part of future multivalent or variant-specific vaccines.
5. How soon will nasal vaccines be globally available?
Launches are projected between 2025–2026 with licensing in place for international production and access.
References & Data Sources
Internal pharma market intelligence & proprietary modeling
Open-access data from Weizmann Institute, Hebrew University
Israeli Ministry of Health & CEPI partnership communications
Patent databases and WHO global vaccine development frameworks

