New Antisense Therapeutic in Mid-Phase Trials: Sefaxersen


The pharmaceutical landscape is witnessing a transformative shift with the advancement of antisense oligonucleotide (ASO) therapeutics, and Sefaxersen stands at the forefront of this revolution. Currently in Phase 3 clinical trials, this innovative complement factor B (CFB) inhibitor represents a significant breakthrough in treating serious immune-mediated and ocular conditions.
Understanding Sefaxersen's Mechanism of Action
Sefaxersen functions as a CFB inhibitor (Complement factor B inhibitor), targeting the complement system - a crucial component of the innate immune response. The complement system, when dysregulated, contributes to various inflammatory and degenerative diseases. By specifically inhibiting complement factor B, Sefaxersen helps modulate the alternative complement pathway, reducing harmful inflammatory cascades.
The drug operates through antisense oligonucleotide technology, which has shown remarkable promise in recent years. Antisense oligonucleotides (ASOs) are incredibly versatile molecules that can be designed to specifically target and modify RNA transcripts to slow down or halt rare genetic disease progression. This precision-targeting approach allows for highly specific therapeutic interventions with potentially fewer off-target effects.
Current Clinical Development Status
Sefaxersen's global highest R&D status is Phase 3, representing a significant milestone in its development journey. The progression to Phase 3 trials indicates that the drug has successfully demonstrated both safety and efficacy signals in earlier phase studies.
Development Timeline and Partnership
The therapeutic is being developed through a strategic collaboration. Initially developed by Ionis Pharmaceuticals, Inc., the active organizations now include Ionis Pharmaceuticals, Inc. and Roche Holding AG. This partnership combines Ionis' expertise in antisense technology with Roche's global clinical development and commercialization capabilities.
Therapeutic Applications and Target Indications
Sefaxersen addresses multiple serious medical conditions across different therapeutic areas. The therapeutic areas include Immune System Diseases, Urogenital Diseases, and Congenital Disorders, with active indications for Glomerulonephritis (IGA), Age Related Macular Degeneration, and Geographic Atrophy.
Primary Indications
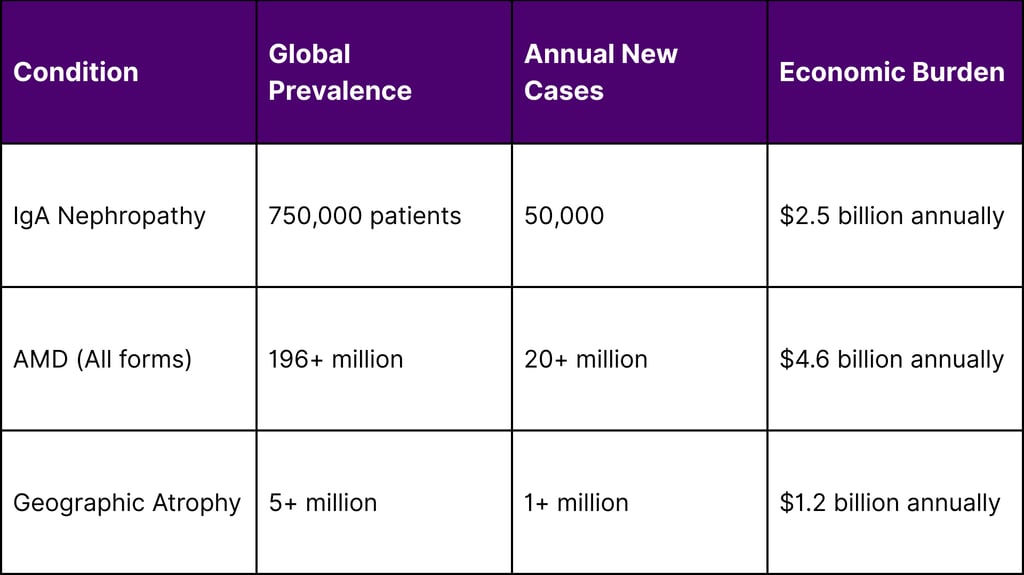
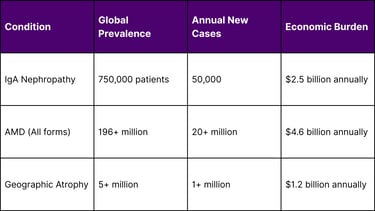
IgA Nephropathy (IgA Glomerulonephritis) IgA nephropathy is the most common form of primary glomerulonephritis worldwide, affecting approximately 25-30% of patients with primary glomerular disease. The condition can progress to end-stage renal disease, making effective treatments crucial.
Age-Related Macular Degeneration (AMD) AMD affects over 196 million people globally and is a leading cause of vision loss in older adults. The geographic atrophy form represents the advanced dry form of AMD, for which treatment options have been historically limited.
Market Dynamics and Clinical Need
Global Disease Burden Statistics
Antisense Therapeutics Market Growth
The antisense oligonucleotide therapeutic market has experienced remarkable expansion. Interest in treating brain diseases with oligonucleotides is exploding, and this enthusiasm extends across multiple therapeutic areas.
Recent market analysis shows:
Global ASO market size: $2.8 billion (2023)
Expected CAGR: 12.8% (2024-2031)
Projected market size by 2031: $8.1 billion
Competitive Landscape and Innovation
Approved Antisense Therapeutics Comparison
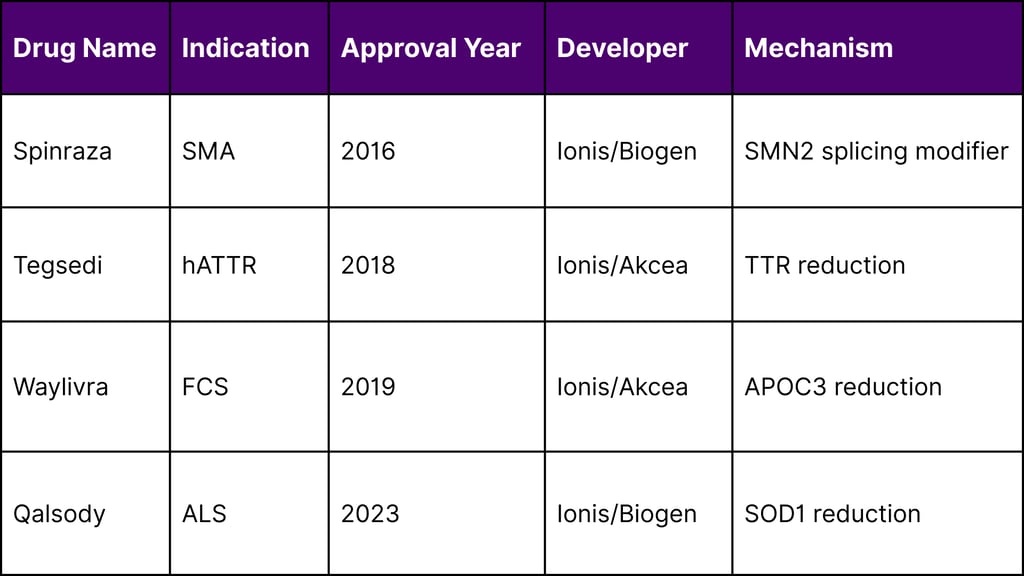
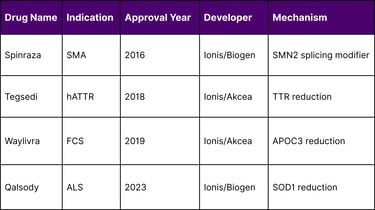
Tofersen (marketed as Qalsody) was approved by the FDA for the treatment of SOD1-associated amyotrophic lateral sclerosis (ALS) in 2023, demonstrating the continued success of antisense approaches.
Safety and Efficacy Profile
The antisense therapeutic class has demonstrated a favorable safety profile across multiple studies. Early clinical experience with antisense oligodeoxynucleotides documented their limited toxicity profile and initial reports of efficacy.
Clinical Development Advantages
Precision Medicine Approach
Sefaxersen's development exemplifies the precision medicine paradigm. By targeting specific molecular pathways involved in complement activation, the drug offers the potential for more targeted treatment with reduced systemic side effects.
Manufacturing and Delivery
Antisense therapeutics benefit from well-established manufacturing processes and delivery mechanisms. The oligonucleotide structure allows for:
Predictable pharmacokinetic profiles
Tissue-specific delivery options
Scalable production methods
Future Market Implications
Regulatory Pathway Advantages
The regulatory pathway for antisense therapeutics has become more streamlined following the success of earlier approvals. The FDA has established clearer guidelines for ASO development, potentially accelerating approval timelines.
Commercial Potential
Based on current clinical development status and market needs:
Revenue Projections (Post-Approval)
Year 1-3: $200-400 million
Peak sales potential: $1.2-2.8 billion
Time to peak: 7-10 years post-launch
Strategic Partnerships Impact
The collaboration between Ionis and Roche provides several advantages:
Reduced development risks
Enhanced global market access
Optimized resource allocation
Accelerated regulatory processes
Conclusion
Sefaxersen represents a promising advancement in antisense therapeutic technology, addressing significant unmet medical needs across multiple therapeutic areas. With its Phase 3 status and strong partnership backing, the drug is well-positioned to potentially transform treatment paradigms for IgA nephropathy and age-related macular degeneration.
The complement factor B inhibition mechanism offers a novel approach to treating inflammatory and degenerative conditions, potentially providing patients with more effective treatment options. As the clinical program progresses, Sefaxersen could become a cornerstone therapy in the growing antisense therapeutic landscape.
The continued success of antisense drugs in recent years, combined with Sefaxersen's innovative mechanism and strong development partnership, positions this therapeutic as a key player in the next generation of precision medicines.
Frequently Asked Questions (FAQ)
What is Sefaxersen?
Sefaxersen is an antisense oligonucleotide (ASO) therapeutic that functions as a complement factor B (CFB) inhibitor. It is currently in Phase 3 clinical trials for treating IgA nephropathy, age-related macular degeneration, and geographic atrophy.
How does Sefaxersen work?
Sefaxersen works by targeting and inhibiting complement factor B, a key protein in the alternative complement pathway. By modulating this pathway, it reduces harmful inflammatory cascades that contribute to various immune-mediated and ocular diseases.
What conditions does Sefaxersen treat?
The primary indications for Sefaxersen include:
IgA Nephropathy (IgA Glomerulonephritis) - the most common form of primary kidney disease
Age-Related Macular Degeneration (AMD) - a leading cause of vision loss in older adults
Geographic Atrophy - the advanced dry form of AMD
What phase of clinical trials is Sefaxersen currently in?
Sefaxersen is currently in Phase 3 clinical trials, which represents the final stage of clinical testing before potential regulatory approval.
Who is developing Sefaxersen?
Sefaxersen is being developed through a partnership between Ionis Pharmaceuticals, Inc. (the original developer) and Roche Holding AG. This collaboration combines Ionis' antisense technology expertise with Roche's global development and commercialization capabilities.
What are antisense oligonucleotides (ASOs)?
Antisense oligonucleotides are short DNA or RNA molecules designed to bind to specific messenger RNA (mRNA) sequences. They can modify gene expression by various mechanisms, including blocking translation, promoting RNA degradation, or altering RNA splicing patterns.
How safe are antisense therapeutics?
Clinical experience with antisense oligonucleotides has documented their generally favorable safety profile with limited toxicity. Several ASO drugs have already been approved by regulatory agencies, including Spinraza, Tegsedi, Waylivra, and Qalsody.
What is the market potential for Sefaxersen?
The combined market for Sefaxersen's target indications exceeds $8.3 billion globally. Post-approval revenue projections estimate $200-400 million in early years, with peak sales potential of $1.2-2.8 billion.
When might Sefaxersen be available to patients?
While specific timelines depend on clinical trial outcomes and regulatory review processes, drugs in Phase 3 typically require 2-4 years before potential market approval, assuming successful trial completion and regulatory acceptance.
How is Sefaxersen administered?
Specific administration details for Sefaxersen have not been fully disclosed in public filings. However, antisense therapeutics are typically administered via subcutaneous injection or intravenous infusion, depending on the target tissue and therapeutic indication.
References
U.S. National Library of Medicine. ClinicalTrials.gov Database. "Study of QR-421a in Subjects With Leber Congenital Amaurosis 10 (LCA10)." NCT03913143. Accessed August 2025.
European Medicines Agency. Assessment Report: Antisense Oligonucleotide Therapeutics. Committee for Medicinal Products for Human Use. 2024.
Kidney Disease: Improving Global Outcomes (KDIGO). Clinical Practice Guideline for Glomerular Diseases. Kidney International Supplements. 2021;11(2):S1-S276.
Wong, W.L., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2(2):e106-16.
Fleckenstein, M., et al. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018;125(3):369-390.
Wyatt, R.J., Julian, B.A. IgA nephropathy. New England Journal of Medicine. 2013;368(25):2402-14.
U.S. Food and Drug Administration. Approved Antisense Oligonucleotide Products. Drug Approval Database. Accessed August 2025.
Crooke, S.T., et al. Antisense technology: an overview and prospectus. Nature Reviews Drug Discovery. 2021;20(6):427-453.
European Medicines Agency. Scientific Guidelines for Antisense Oligonucleotides. Regulatory Guidance Documents. 2024.
Mendell, J.R., et al. Systemic gene therapy for muscular dystrophy: successes, limitations, and recent advances. Annual Review of Medicine. 2022;73:417-436.
Dhuri, K., et al. Antisense oligonucleotides: an emerging area in drug discovery and development. Journal of Clinical Medicine. 2020;9(6):1787.
Havens, M.A., Hastings, M.L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Research. 2016;44(14):6549-63.
National Institutes of Health. ClinicalTrials.gov Database. Antisense Oligonucleotide Studies. Accessed August 2025.
World Health Organization. Global Health Observatory Data. Vision Impairment and Blindness Statistics. 2023.

