New FDA approvals for Belzutifan (Welireg)


The pharmaceutical landscape witnessed significant developments in 2024-2025 with multiple FDA approvals for Belzutifan (Welireg®), establishing this first-in-class HIF-2α inhibitor as a groundbreaking therapeutic option across several cancer indications. These approvals represent critical milestones in addressing unmet medical needs in oncology, particularly for rare tumors with limited treatment options.
Recent FDA Approval Timeline and Indications
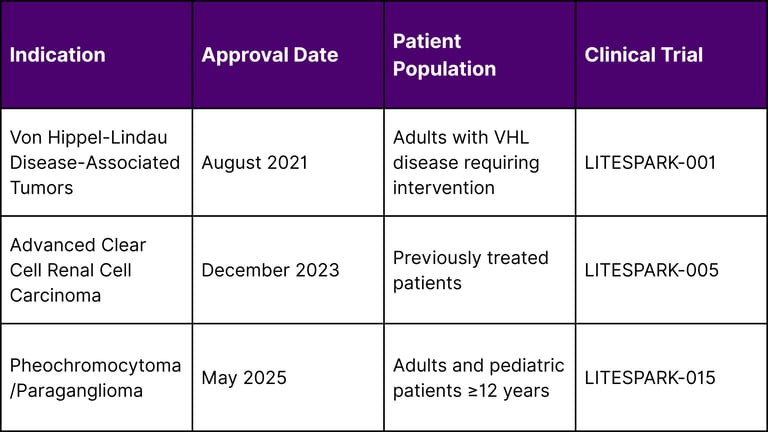
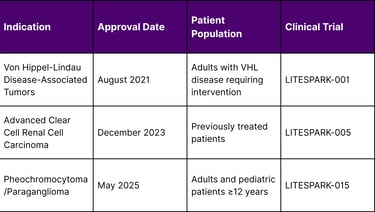
On May 14, 2025, the Food and Drug Administration approved Belzutifan (Welireg, Merck & Co., Inc.) for adult and pediatric patients 12 years and older with locally advanced, unresectable, or metastatic pheochromocytoma or paraganglioma (PPGL), marking the first oral treatment authorized for these tumors. This latest approval follows the December 14, 2023 FDA approval for patients with advanced renal cell carcinoma (RCC) following treatment with a PD-1/PD-L1 inhibitor and a VEGF tyrosine kinase inhibitor.
Current FDA-Approved Indications for Belzutifan
Clinical Efficacy Data and Market Impact
The expanding approval portfolio is supported by robust clinical data across multiple Phase II and III trials. The most significant efficacy data comes from the LITESPARK-005 study, which demonstrated superior outcomes compared to standard therapy.
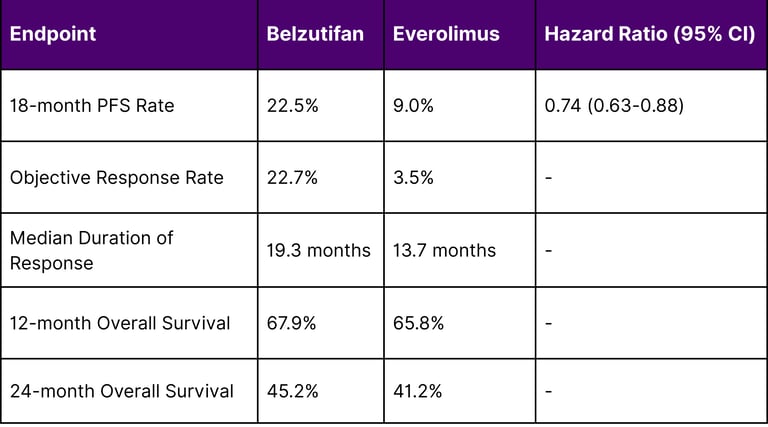
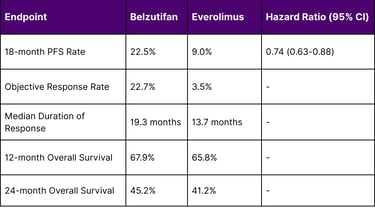
LITESPARK-005 Trial Results (Advanced RCC)
At the landmark 18 months analysis, 22.5% of patients remained progression-free with belzutifan, compared to 9% with everolimus (HR: 0.74, 95% CI: 0.63–0.88). The trial met its primary endpoint with statistically significant improvements in progression-free survival.
Key Efficacy Metrics:
LITESPARK-015 Trial Results (PPGL)
In cohort A1 (n = 72), the agent demonstrated a confirmed overall response rate of 26% (95% CI, 17%-38%) in patients with advanced pheochromocytoma and paraganglioma, establishing efficacy in this rare tumor type with no previously approved therapies.
Market Analysis and Commercial Implications
Market Size and Growth Projections
Renal Cell Carcinoma Market:
Global RCC market size: $4.2 billion (2023)
Projected CAGR: 8.5% (2024-2030)
Advanced RCC segment: ~30% of total RCC market
Pheochromocytoma/Paraganglioma Market:
Annual incidence: 3-8 per million population
Estimated global market: $150-200 million
Significant unmet need with no prior approved therapies
Competitive Landscape Position
Belzutifan's unique mechanism of action as a HIF-2α inhibitor differentiates it from existing therapies:
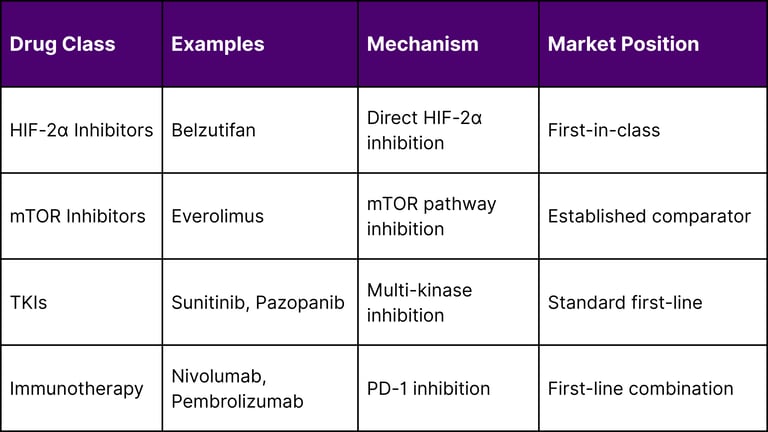
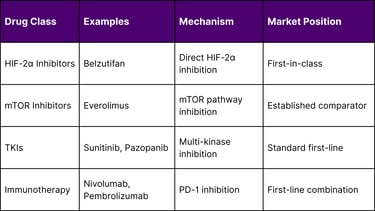
Safety Profile and Tolerability
The safety profile across clinical trials demonstrates manageable adverse events with a distinct toxicity pattern compared to other targeted therapies:
Most Common Adverse Events (≥20%):
Anemia (89%)
Fatigue (66%)
Dyspnea (40%)
Dizziness (36%)
Nausea (34%)
Grade 3-4 Adverse Events:
Anemia (39%)
Hypoxia (8%)
Fatigue (7%)
Regulatory Strategy and Priority Review
The FDA granted priority review to the drug's supplemental new drug application (sNDA) for use in PPGL on January 27, 2025, with a target action date of May 26, 2025. This expedited timeline reflects the significant unmet medical need in these rare tumor types.
Priority Review Criteria Met:
First therapy approved for advanced PPGL
Addresses unmet medical need in rare disease
Demonstrates meaningful clinical benefit
Future Development Pipeline
The success of belzutifan across multiple indications suggests potential for further expansion:
Ongoing Studies:
Combination therapies with immunotherapy agents
Earlier-line treatment in RCC
Additional rare VHL-associated tumors
Pediatric expansion studies
Market Access and Healthcare Economics
Pricing Strategy Considerations:
Orphan drug designation benefits
Value-based pricing models
Payer coverage policies for rare diseases
Patient assistance programs
Healthcare Utilization Impact:
Reduced need for surgical interventions in VHL patients
Oral administration improving patient convenience
Potential for outpatient management
Global Regulatory Status
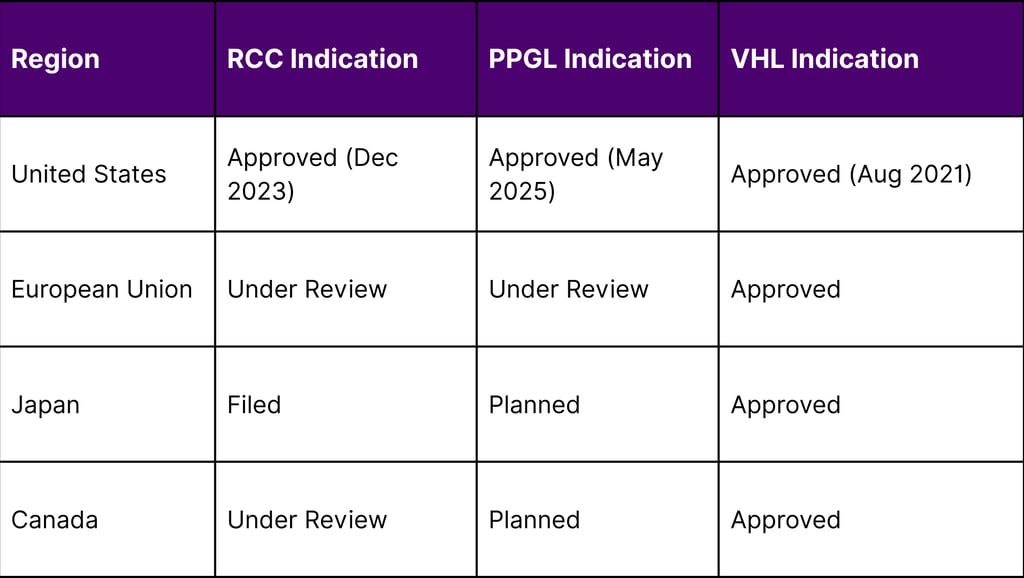
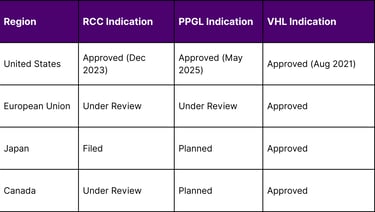
Key Success Factors and Market Drivers
First-in-Class Advantage: Novel mechanism of action provides competitive differentiation
Robust Clinical Data: Strong efficacy signals across multiple tumor types
Unmet Medical Need: Addresses gaps in rare disease treatment options
Oral Formulation: Improves patient convenience and quality of life
Manageable Safety Profile: Distinct from existing targeted therapies
Challenges and Risk Factors
Market Access Challenges:
High development costs for rare disease indications
Complex reimbursement landscape
Limited patient populations for commercial success
Clinical Challenges:
Long-term safety data still emerging
Need for biomarker-driven patient selection
Competition from emerging HIF pathway inhibitors
Conclusion
The recent FDA approvals for Belzutifan represent a significant advancement in targeted cancer therapy, particularly for patients with rare tumors and limited treatment options. The drug's success across multiple indications validates the HIF-2α pathway as a therapeutic target and establishes belzutifan as a cornerstone therapy in its approved indications.
For pharmaceutical companies and investors, Belzutifan's trajectory demonstrates the value of precision medicine approaches in rare diseases, with the potential for meaningful clinical benefit translating to commercial success despite small patient populations.
Frequently Asked Questions (FAQ)
Q1: What makes Belzutifan different from other cancer treatments?
A: Belzutifan is the first FDA-approved drug that directly inhibits HIF-2α (hypoxia-inducible factor 2-alpha), a protein that helps cancer cells survive in low-oxygen environments. This unique mechanism sets it apart from traditional chemotherapy, immunotherapy, and other targeted therapies.
Q2: Which patients are eligible for Belzutifan treatment?
A: Currently, Belzutifan is approved for three main patient groups: adults with Von Hippel-Lindau disease-associated tumors requiring intervention, adults with previously treated advanced clear cell renal cell carcinoma, and adults and pediatric patients 12 years and older with locally advanced, unresectable, or metastatic pheochromocytoma or paraganglioma.
Q3: How effective is Belzutifan compared to existing treatments?
A: In advanced renal cell carcinoma, clinical trials showed that 22.5% of patients remained progression-free at 18 months with Belzutifan compared to 9% with Everolimus. The objective response rate was 22.7% versus 3.5% for the comparator drug.
Q4: What are the most common side effects?
A: The most common side effects include anemia (89% of patients), fatigue (66%), shortness of breath (40%), dizziness (36%), and nausea (34%). Most side effects are manageable with appropriate monitoring and supportive care.
Q5: Is Belzutifan available globally?
A: Belzutifan has received approvals in the United States for all three indications. It's also approved in the European Union and Japan for Von Hippel-Lindau disease. Regulatory submissions are ongoing or planned for other indications in various international markets.
Q6: How is Belzutifan administered?
A: Belzutifan is taken orally as a tablet, typically once daily. This oral administration offers convenience compared to intravenous treatments and allows patients to take their medication at home.
Q7: What is the significance of the pheochromocytoma/paraganglioma approval?
A: This approval is particularly significant because it represents the first FDA-approved therapy for advanced pheochromocytoma or paraganglioma, addressing a major unmet medical need for patients with these rare tumors.
Q8: Are there ongoing studies for Belzutifan?
A: Yes, multiple clinical trials are ongoing to evaluate Belzutifan in combination with other therapies, in earlier lines of treatment, and in additional tumor types. These studies may lead to expanded indications in the future.
References
U.S. Food and Drug Administration. FDA approves Belzutifan for pheochromocytoma or paraganglioma. May 14, 2025.
Choueiri TK, et al. Belzutifan versus everolimus in participants with previously treated advanced clear cell renal cell carcinoma (LITESPARK-005): a randomised, open-label, phase 3 trial. Lancet Oncol. 2024;25(1):119-130.
Jonasch E, et al. Phase I LITESPARK-001 study of Belzutifan for advanced solid tumors: Extended 41-month follow-up in the clear cell renal cell carcinoma cohort. Eur J Cancer. 2023;195:113360.
Merck & Co., Inc. FDA Approves Merck's WELIREG® (Belzutifan) for the Treatment of Adults and Pediatric Patients 12 Years and Older With Locally Advanced, Unresectable, or Metastatic Pheochromocytoma or Paraganglioma (PPGL). News release. May 14, 2025.
Clinical Trials.gov. A Study of MK-6482 in Participants With Von Hippel-Lindau Disease-Associated Tumors (MK-6482-015/LITESPARK-015). NCT04924075.
Srinivasan R, et al. FDA Approval Summary: Belzutifan for Patients with Advanced Renal Cell Carcinoma. Clin Cancer Res. 2024;30(22):5066-5071.
European Medicines Agency. Assessment report: Welireg (Belzutifan). EMA/CHMP/624399/2022.
National Cancer Institute. Pheochromocytoma and Paraganglioma Treatment (PDQ®)–Health Professional Version. Updated 2024.
Motzer RJ, et al. Belzutifan monotherapy for VHL disease-associated renal cell carcinoma. N Engl J Med. 2021;385(22):2036-2046.

