Precision Medicine in Oncology


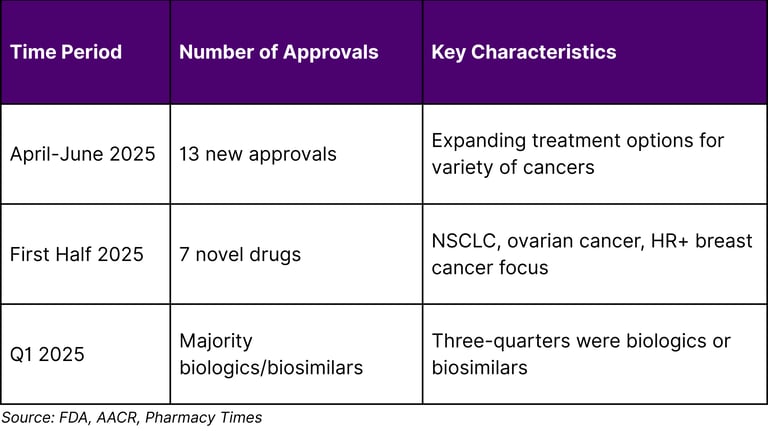
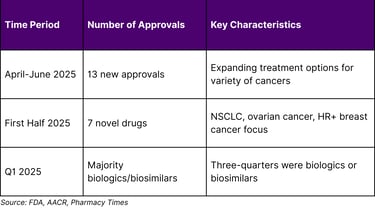
The precision medicine oncology landscape is experiencing unprecedented transformation, fundamentally shifting cancer treatment from generalized approaches to personalized therapeutic strategies. Researchers are now identifying the molecular fingerprints of various cancers and using them to divide cancer's once-broad categories into far more precise types and subtypes. This evolution is supported by robust regulatory momentum, with the FDA issuing 13 new oncology approvals from April-June 2025 and 7 novel oncology drugs approved in the first half of 2025.
This comprehensive analysis explores the intricate market segmentation strategies that are reshaping oncology therapeutics, examining how pharmaceutical companies, healthcare providers, and research institutions are leveraging molecular profiling, biomarker identification, and patient stratification to deliver more effective cancer treatments.
Market Overview and Regulatory Landscape
The precision oncology field represents a paradigm shift in cancer care delivery. The mantra of precision medicine is "divide and conquer." That is, divide cancers into molecular subtypes, and treat them with drugs that target the abnormal biological mechanisms that define each subtype. This approach is supported by continuous regulatory approvals and advancing scientific understanding.
Recent FDA Regulatory Activity (2025)
The regulatory landscape demonstrates strong momentum in precision oncology approvals:
Notable 2025 Approvals
Key Recent Approvals:
Datopotamab deruxtecan-dlnk (Datroway) - June 23, 2025: Accelerated approval for EGFR-mutated NSCLC
Nivolumab (Opdivo), ipilimumab (Yervoy), larotrectinib (Vitrakvi) - April 2025
Key Market Segmentation Strategies
1. Molecular Biomarker-Based Segmentation
Comprehensive molecular profiling of the tumor itself is necessary to determine the presence or absence of certain targetable abnormalities or biomarkers. This forms the foundation of precision oncology segmentation.
Primary Biomarker Categories:
Predictive Biomarkers: Treatment response indicators (HER2, EGFR)
Prognostic Biomarkers: Disease progression predictors (BRCA1/2)
Pharmacodynamic Biomarkers: Drug activity measures (PD-L1)
Safety Biomarkers: Adverse reaction indicators
2. Cancer Type-Specific Therapeutic Targeting
Modern precision oncology segments patients based on specific cancer types and their unique molecular characteristics:
Major Cancer Categories in Precision Medicine:
Non-Small Cell Lung Cancer (NSCLC): Leading in targeted therapy development
Breast Cancer: Hormone receptor and HER2 stratification
Hematological Malignancies: CAR-T and immunotherapy focus
Ovarian Cancer: PARP inhibitor applications
Colorectal Cancer: Microsatellite instability targeting
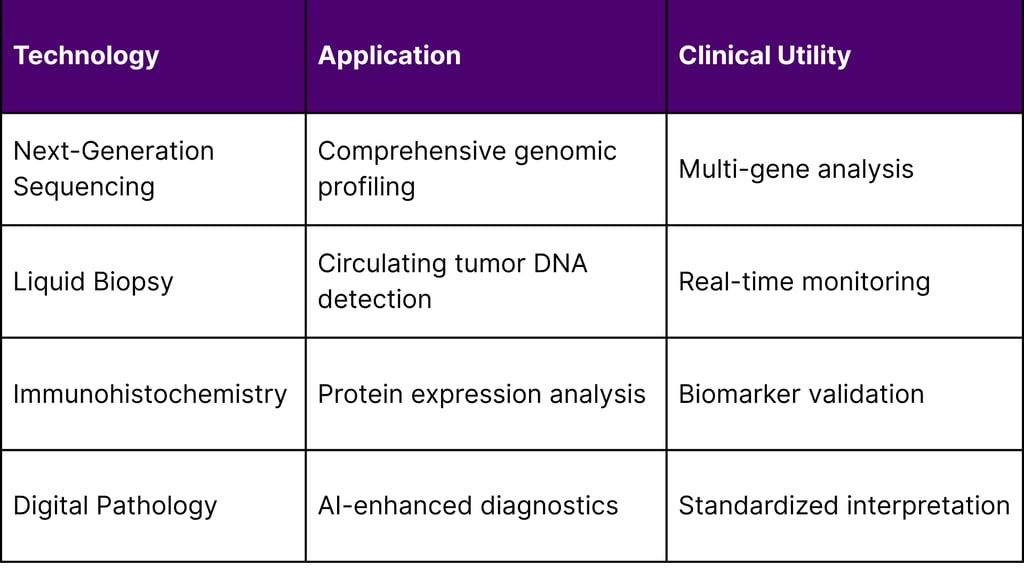
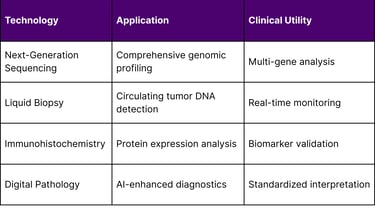
3. Technology Platform Integration
Multiple technological platforms enable comprehensive patient stratification:
4. Therapeutic Modality Classification
The utility of molecular profiling of cancer to identify actionable aberrations continues to expand, leading to diverse therapeutic approaches:
Primary Therapeutic Categories:
Targeted Small Molecules: Kinase inhibitors, metabolic modulators
Monoclonal Antibodies: Immune checkpoint inhibitors, growth factor targeting
Antibody-Drug Conjugates: Targeted delivery systems
Cell-Based Therapies: CAR-T, tumor-infiltrating lymphocytes
Combination Approaches: Multi-target strategies
Patient Stratification Methodologies
Advanced Diagnostic Integration
Theranostics—using diagnostic tools like positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI) with therapies—is experiencing its moment in the spotlight, enhancing patient selection precision.
Multi-Modal Stratification Approaches:
Genomic Profiling: Mutation, amplification, and fusion detection
Transcriptomic Analysis: Gene expression signatures
Proteomic Assessment: Protein biomarker quantification
Metabolomic Evaluation: Metabolic pathway analysis
Artificial Intelligence Integration
When radiologists add artificial intelligence (AI) to the mix, they can analyze images like never before. AI helps us address human computation limits by connecting different data sources.
AI Applications in Precision Oncology:
Image Analysis: Enhanced diagnostic accuracy
Pattern Recognition: Molecular subtype identification
Treatment Prediction: Response probability modeling
Biomarker Discovery: Novel target identification
Clinical Implementation and Quality Assurance
Molecular Tumor Boards
Healthcare institutions are implementing specialized multidisciplinary teams to optimize precision medicine decision-making:
Key Components:
Molecular Pathologists: Genomic interpretation
Medical Oncologists: Treatment selection
Bioinformaticians: Data analysis and interpretation
Pharmacists: Drug interaction assessment
Genetic Counselors: Patient education and support
Standardization Efforts
The promise of precision medicine will only be fully realized if the research community can adapt its clinical trials methodology to study molecularly characterized tumors instead of traditional histologic classification.
Quality Metrics:
Turnaround Time: Diagnostic to treatment initiation
Accuracy Standards: Analytical and clinical validity
Coverage Assessment: Actionable target identification
Clinical Utility: Patient outcome improvements
Regulatory Framework and Compliance
FDA Approval Pathways
The FDA has established multiple pathways to accelerate precision medicine development:
Expedited Programs:
Breakthrough Therapy Designation: Significant clinical advantage
Accelerated Approval: Surrogate endpoint validation
Priority Review: Shortened evaluation timeline
Fast Track Designation: Unmet medical need addressing
Companion Diagnostics Integration
Accelerated approvals for malignant hematology and oncology indications have postmarketing requirements for ongoing clinical trials to verify clinical benefit.
Regulatory Requirements:
Co-development: Therapeutic and diagnostic validation
Clinical Validation: Analytical and clinical performance
Quality Standards: Manufacturing and testing protocols
Post-market Surveillance: Real-world performance monitoring
Current Challenges and Solutions
Technical Implementation Barriers
Primary Challenges:
Tumor Heterogeneity: Intra-tumoral genetic diversity
Temporal Evolution: Dynamic tumor genetics over time
Sample Quality: Adequate tissue for analysis
Interpretation Complexity: Variant of uncertain significance
Healthcare System Integration
Infrastructure Requirements:
Laboratory Capabilities: Molecular testing platforms
Informatics Systems: Data management and interpretation
Clinical Expertise: Specialized personnel training
Quality Assurance: Standardized protocols and procedures
Economic Considerations
Cost-Effectiveness Factors:
Diagnostic Testing: Comprehensive genomic profiling costs
Therapeutic Expenses: Targeted therapy pricing
Infrastructure Investment: Technology platform implementation
Training Requirements: Personnel education and certification
Emerging Opportunities and Innovation
Next-Generation Therapeutic Targets
Research pipelines are expanding to include novel molecular targets:
Emerging Target Categories:
Epigenetic Modifiers: DNA methylation, histone modifications
Metabolic Pathways: Tumor metabolism disruption
Tumor Microenvironment: Immune and stromal targeting
Synthetic Lethality: Cancer-specific vulnerabilities
Technology Convergence
Our understanding of molecular mechanisms underlying cancer development and evolution have evolved rapidly, and variation from patient to another is now widely recognized.
Integration Opportunities:
Multi-Omics Analysis: Genomics, proteomics, metabolomics
Real-World Evidence: Electronic health record integration
Wearable Technology: Continuous patient monitoring
Telemedicine Platforms: Remote consultation capabilities
Strategic Recommendations
For Healthcare Institutions
Implementation Strategy:
Molecular Testing Infrastructure: Establish comprehensive genomic profiling capabilities
Multidisciplinary Teams: Create molecular tumor boards
Quality Assurance Programs: Implement standardized protocols
Staff Education: Develop precision medicine training programs
For Pharmaceutical Companies
Development Strategy:
Companion Diagnostics: Co-develop therapeutic and diagnostic approaches
Biomarker Discovery: Invest in novel target identification
Combination Therapies: Develop synergistic treatment approaches
Real-World Evidence: Generate post-market effectiveness data
For Diagnostic Companies
Innovation Focus:
Platform Integration: Develop comprehensive testing solutions
Turnaround Time: Optimize workflow efficiency
AI Integration: Enhance interpretation accuracy
Quality Standards: Maintain regulatory compliance
Future Directions and Market Evolution
Expanding Applications
Advances in precision oncology continue evolving with predictive molecular events used to select patients who will benefit clinically from treatment.
Growth Areas:
Early Detection: Screening and prevention applications
Minimal Residual Disease: Treatment monitoring
Immunotherapy Selection: Response prediction
Combination Strategies: Multi-target approaches
Global Access and Equity
Key Considerations:
Healthcare Disparities: Rural and underserved populations
International Implementation: Global regulatory harmonization
Cost Accessibility: Value-based care models
Educational Resources: Provider training programs
Conclusion
The precision medicine oncology field continues to evolve rapidly, driven by advancing scientific understanding, regulatory support, and technological innovation. While doctors have historically made recommendations based on expected response of an average patient, the current paradigm enables truly personalized therapeutic approaches.
Success in this dynamic environment requires integrated strategies combining technological innovation, regulatory compliance, quality assurance, and patient-centered care. The continuous stream of FDA approvals, advancing diagnostic capabilities, and expanding therapeutic options create unprecedented opportunities for improving cancer patient outcomes.
Organizations that effectively integrate molecular profiling, AI-enhanced analytics, and multidisciplinary care delivery will be optimally positioned to capitalize on the evolving precision oncology landscape. The future emphasizes not just technological advancement, but practical implementation of personalized medicine approaches that deliver measurable clinical benefits to cancer patients worldwide.
Frequently Asked Questions (FAQ)
Q1: How many oncology drugs were approved by the FDA in 2025?
A1: The FDA approved 7 novel drugs for oncologic conditions in the first half of 2025, with 13 new oncology approvals from April-June 2025 alone.
Q2: What is the fundamental approach of precision medicine in oncology?
A2: The mantra of precision medicine is "divide and conquer" - divide cancers into molecular subtypes and treat them with drugs targeting abnormal biological mechanisms defining each subtype.
Q3: What role does molecular profiling play in precision oncology?
A3: Comprehensive molecular profiling of the tumor is necessary to determine the presence or absence of certain targetable abnormalities or biomarkers.
Q4: How is artificial intelligence being integrated into precision medicine?
A4: AI helps address human computation limits by connecting different data sources, enabling radiologists to analyze images like never before.
Q5: What are theranostics in precision oncology?
A5: Theranostics involves using diagnostic tools like PET, CT, and MRI with therapies like chemotherapy, gene therapy, and radiation.
Q6: What challenges exist in implementing precision medicine?
A6: The research community must adapt clinical trials methodology to study molecularly characterized tumors instead of traditional histologic classification.
Q7: How has cancer understanding evolved?
A7: Understanding of molecular mechanisms underlying cancer development and evolution have evolved rapidly, and variation from one patient to another is now widely recognized.
Q8: What is required for successful precision medicine implementation?
A8: Success requires comprehensive molecular profiling, multidisciplinary care teams, quality assurance programs, and integration of advanced diagnostic technologies with therapeutic decision-making.
Q9: What are the major cancer types benefiting from precision medicine?
A9: Recent FDA approvals focus on non-small cell lung cancer, ovarian cancer, and hormone receptor-positive breast cancer, among others.
Q10: How do FDA approval pathways support precision medicine?
A10: The FDA provides accelerated approvals with postmarketing requirements for ongoing clinical trials to verify clinical benefit.
References
National Institutes of Health. (2025). "Precision Oncology." NIH Turning Discovery into Health.
National Institutes of Health. (2025). "The Promise of Precision Medicine."
National Cancer Institute. "National Cancer Institute's Precision Medicine Initiatives for the new National Clinical Trials Network."
National Cancer Institute. "How Genomics Is Shaping Precision Medicine in Oncology."
PubMed. (2024). "Precision Oncology: 2024 in Review."
PMC - PubMed Central. "The Future of Precision Oncology."
PMC - PubMed Central. "Precision Medicine in Oncology: A Review of Multi-Tumor Actionable Molecular Targets."
PubMed. "Precision cancer medicine: the future is now, only better."
American Association for Cancer Research. (2025). "FDA Approvals in Oncology: April-June 2025." AACR Blog.
U.S. Food and Drug Administration. (2025). "FDA grants accelerated approval to datopotamab deruxtecan-dlnk for EGFR-mutated non-small cell lung cancer."

