Rare Disease Market Dynamics


The pharmaceutical industry is witnessing an unprecedented transformation where rare diseases—conditions affecting fewer than 200,000 people in the United States—are driving some of the most lucrative market opportunities. This paradox of small patient populations generating billion-dollar revenues is reshaping drug development strategies and creating new investment paradigms across the global healthcare sector.
The Expanding Rare Disease Universe
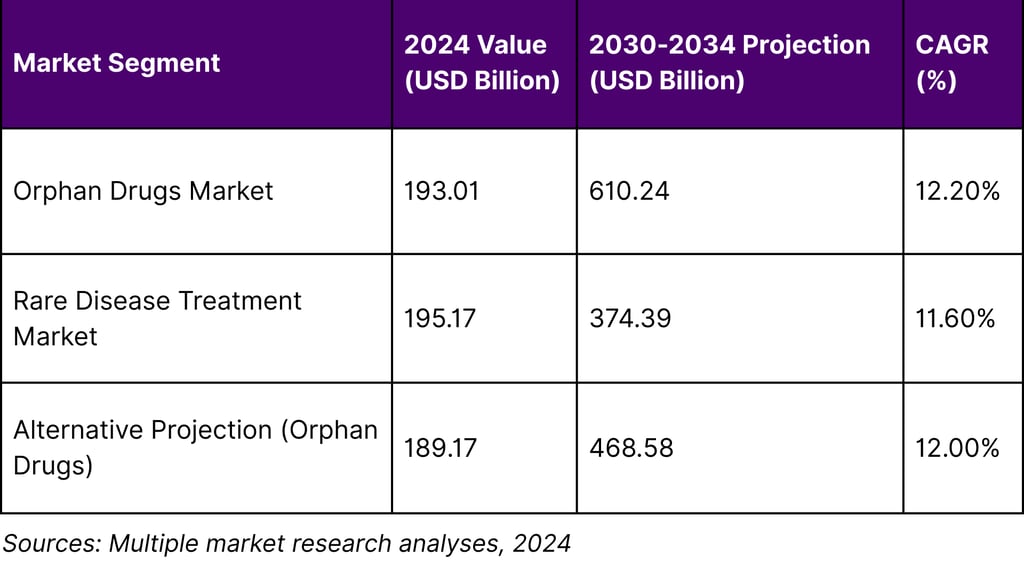
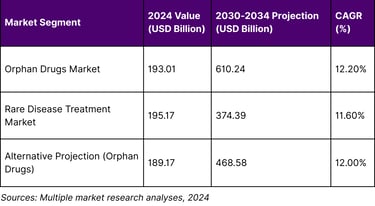
The rare disease therapeutic market has evolved into a financial powerhouse that defies traditional pharmaceutical economics. The global orphan drugs market size was surpass to USD 193.01 billion in 2024 and is projected to reach around USD 610.24 billion by 2034 with a CAGR of 12.20%. This remarkable growth trajectory positions rare disease therapeutics among the fastest-growing segments in pharmaceutical development.
The scale of this market opportunity becomes even more compelling when examining the broader treatment landscape. The global rare diseases treatment market size was estimated at USD 195,174.8 million in 2024 and is projected to reach USD 374,391.0 million by 2030, growing at a CAGR of 11.6% from 2025 to 2030, indicating sustained momentum across multiple market analyses.
Market Size and Growth Projections
Regulatory Environment Driving Innovation
The regulatory landscape has become increasingly favorable for rare disease drug development. 26 of 50, or 52% of our novel drug approvals were approved for rare diseases according to the FDA's 2024 annual report, highlighting the significant focus on addressing unmet medical needs in rare conditions.
The FDA's commitment to rare disease therapeutics is further evidenced by the efficiency of their approval processes. 33 of the 50 of CDER's novel drug approvals (66%) used one or more of these expedited programs, which helped bring new therapies to the market sooner. These expedited programs include fast track designation, breakthrough therapy designation, priority review designation, and accelerated approval pathways.
FDA Expedited Programs Utilization (2024)
Fast Track Designations: 44% of novel approvals
Breakthrough Therapy: 36% of novel approvals
Priority Review: 56% of novel approvals
Accelerated Approval: 14% of novel approvals
Overall Expedited Programs: 66% of novel approvals
Economic Factors Behind the Billion-Dollar Opportunity
Premium Pricing Models
Rare disease therapeutics command premium pricing due to several interconnected factors:
1. Limited Market Competition: With small patient populations, multiple competitors rarely enter the same therapeutic space, allowing market exclusivity and higher pricing power.
2. High Unmet Medical Need: Many rare diseases have no existing treatments, creating willingness to pay premium prices for effective therapies among patients, providers, and payers.
3. Regulatory Incentives: Orphan drug designation provides seven years of market exclusivity in the United States, protecting investments from generic competition.
4. Development Cost Recovery: The high cost of clinical development for rare diseases must be recouped from a smaller patient population, necessitating higher per-patient pricing.
Market Access and Reimbursement
Despite small patient populations, rare disease therapeutics often achieve favorable reimbursement due to:
Lack of therapeutic alternatives
Severe disease consequences without treatment
Strong patient advocacy
Regulatory support for innovation
Innovation Patterns and First-in-Class Opportunities
CDER identified 24 of the 50 novel drugs approved (48%) in 2024 as first-in-class, with many addressing rare disease indications. This high percentage of first-in-class drugs in rare diseases reflects the innovative approaches required to address previously untreatable conditions.
Notable 2024 First-in-Class Rare Disease Approvals
Breakthrough Mechanisms:
Cobenfy: First muscarinic-acting drug for schizophrenia
Nemluvio: First treatment for prurigo nodularis
Rezdiffra: First drug for MASH (metabolic-dysfunction associated steatohepatitis)
Xolremdi: First treatment for WHIM syndrome
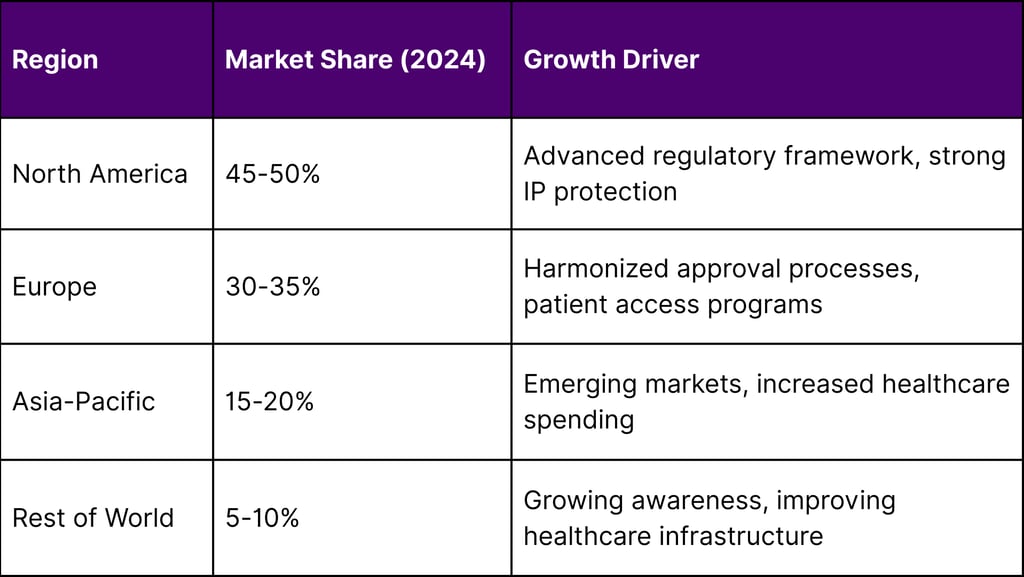
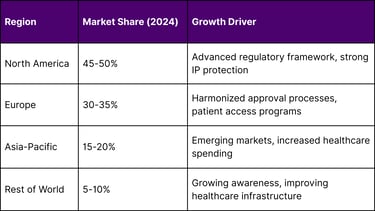
Geographic Market Distribution
Investment and Development Trends
Rising R&D Investment
Pharmaceutical companies are increasingly allocating resources to rare disease programs due to:
Financial Attractiveness:
Higher profit margins compared to traditional therapeutic areas
Reduced commercial risk due to market exclusivity
Faster regulatory timelines and approval rates
Strategic Benefits:
Enhanced company valuation and investor interest
Improved regulatory relationships
Platform technologies applicable to multiple rare conditions
Venture Capital and Biotech Focus
The rare disease sector has attracted significant venture capital investment, with specialized biotech companies leading innovation:
Focused Development Models: Single-asset companies targeting specific rare diseases
Platform Technologies: Gene therapies, antisense oligonucleotides, and other innovative modalities
Patient-Centric Approaches: Direct engagement with patient communities and advocacy groups
Market Challenges and Risk Factors
Development Complexity
Clinical Trial Challenges:
Small patient populations limit trial size and statistical power
Geographic dispersion of patients increases recruitment costs
Natural history studies often required to understand disease progression
Regulatory endpoints may be difficult to establish
Commercial Risks:
Accurate market sizing can be challenging
Patient identification and diagnosis rates may vary significantly
Healthcare system preparedness for ultra-rare treatments
Long-term safety monitoring requirements
Competitive Dynamics
While rare disease markets traditionally have limited competition, several trends are changing this landscape:
Emerging Competition:
Multiple companies targeting the same rare diseases
Platform technologies enabling faster development timelines
Biosimilar competition for successful rare disease biologics
Technology and Innovation Drivers
Advanced Therapeutic Modalities
The rare disease space has become a testing ground for cutting-edge therapeutic approaches:
Gene and Cell Therapies:
One-time treatments for genetic rare diseases
Potentially curative approaches commanding premium pricing
Manufacturing and delivery challenges being addressed
Precision Medicine:
Biomarker-driven patient selection
Personalized treatment approaches
Companion diagnostics development
Digital Health Integration
Technology is enabling new approaches to rare disease management:
Patient registries for natural history data collection
Telemedicine for specialized care access
Digital biomarkers for disease monitoring
AI-powered drug discovery and patient matching
Future Market Outlook
Growth Projections by Therapeutic Area
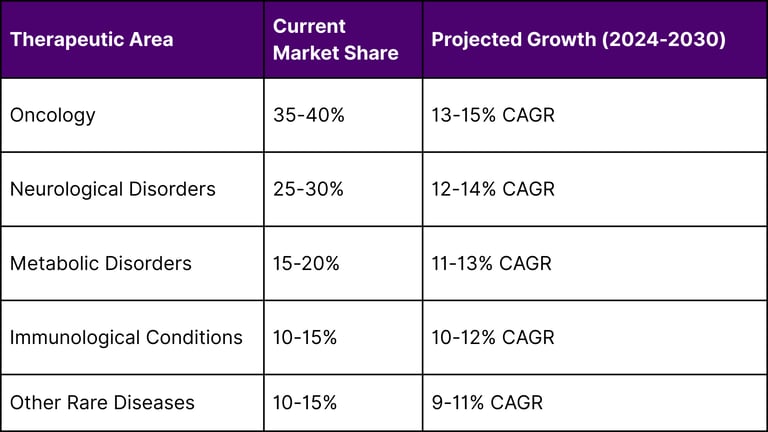
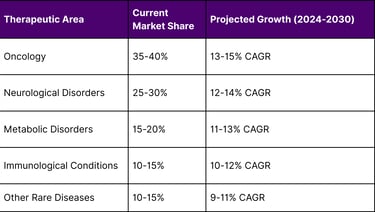
Emerging Opportunities
Next-Generation Targets:
Ultra-rare genetic diseases with well-defined pathways
Pediatric rare diseases with high unmet need
Rare cancer subtypes with specific biomarkers
Neurological conditions with measurable endpoints
Strategic Implications for Industry Stakeholders
For Pharmaceutical Companies
Portfolio Strategy:
Balance rare disease programs with traditional therapeutic areas
Develop platform technologies applicable across multiple rare conditions
Build expertise in regulatory pathways and patient engagement
Partnership Approaches:
Collaborate with patient advocacy organizations
Form alliances with academic medical centers
Engage with specialized rare disease biotechs
For Investors
Investment Considerations:
Strong intellectual property protection
Clear regulatory pathway and FDA engagement
Experienced management teams with rare disease expertise
Robust clinical development plans with appropriate endpoints
Risk Assessment:
Market sizing accuracy and patient identification
Competitive landscape and differentiation
Manufacturing complexity and supply chain risks
Long-term commercial sustainability
Global Market Access and Health Economics
Payer Perspectives
Health technology assessment bodies worldwide are developing specialized frameworks for rare disease evaluations:
Value Assessment Criteria:
Disease severity and unmet medical need
Quality of life improvements
Caregiver burden reduction
Long-term healthcare cost offsets
Access Pathways:
Managed access programs
Risk-sharing agreements
Outcomes-based contracts
International pricing reference considerations
Conclusion
The rare disease pharmaceutical market represents one of the most compelling opportunities in modern healthcare, where small patient populations can indeed generate billion-dollar revenues. The convergence of favorable regulatory environments, premium pricing models, technological innovation, and unmet medical need creates a unique ecosystem for value creation.
Success in this market requires a deep understanding of patient communities, regulatory pathways, and commercial dynamics that differ significantly from traditional pharmaceutical development. Companies that master these specialized requirements while delivering meaningful patient value will continue to drive the remarkable growth trajectory of the rare disease therapeutics market.
The projected growth to over $600 billion by 2034 underscores the transformative potential of this sector, making it essential for industry stakeholders to understand and participate in rare disease market dynamics. As scientific capabilities continue to advance and regulatory frameworks evolve, the rare disease market will remain a critical driver of pharmaceutical innovation and financial performance.
Frequently Asked Questions (FAQs)
Q: What defines a rare disease in pharmaceutical markets?
A: In the United States, a rare disease is defined as a condition affecting fewer than 200,000 people. In Europe, the threshold is 1 in 2,000 people or fewer. These definitions determine eligibility for orphan drug designation and associated regulatory incentives.
Q: How can small patient populations support billion-dollar revenues?
A: Small patient populations can generate substantial revenues through premium pricing models, market exclusivity periods, global expansion, and the high value placed on treatments for previously untreatable conditions. The limited competition and strong unmet medical need justify higher per-patient costs.
Q: What regulatory incentives support rare disease drug development?
A: Key incentives include orphan drug designation (providing 7 years of market exclusivity), tax credits for clinical development costs, expedited regulatory review pathways, and reduced regulatory fees. These benefits help offset the challenges of developing treatments for small patient populations.
Q: What are the main challenges in rare disease drug development?
A: Primary challenges include small clinical trial populations, difficulty in patient identification and recruitment, limited natural history data, regulatory endpoint establishment, geographic patient dispersion, and accurate market sizing difficulties.
Q: How do rare disease markets differ from traditional pharmaceutical markets?
A: Rare disease markets typically feature higher pricing, longer market exclusivity, different commercial strategies focused on specialist physicians, direct patient engagement, specialized distribution channels, and unique health economics value propositions emphasizing unmet medical need.
Q: What role does technology play in rare disease drug development?
A: Technology enables patient registries for natural history studies, AI-powered drug discovery, digital biomarkers for disease monitoring, telemedicine for patient access, and advanced therapeutic modalities like gene and cell therapies specifically suited for rare genetic conditions.
Q: How do investors evaluate rare disease opportunities?
A: Investors assess factors including intellectual property strength, regulatory pathway clarity, management team expertise, clinical development risk, competitive landscape, market sizing accuracy, and commercial sustainability. The risk-return profile differs significantly from traditional drug development investments.
References
FDA Center for Drug Evaluation and Research. (2025). Advancing Health Through Innovation: New Drug Therapy Approvals 2024. U.S. Food and Drug Administration.
National Academy of Sciences. (2024). Advancing Rare Disease Drug Development: Recommendations to Enhance FDA's Role. Washington, DC: The National Academies Press.
U.S. Food and Drug Administration. (2024). Orphan Drug Designations and Approvals Database.
Orphan Disease Center. (2024). Rare Disease Impact Report: Global Perspectives on Unmet Medical Need. University of Pennsylvania.
Government Accountability Office. (2024). Rare Disease Drugs: FDA Has Steps Underway to Strengthen Coordination of Activities Supporting Drug Development (GAO-25-106774).
International Rare Disease Research Consortium. (2024). Global State of Rare Disease Research and Development. Annual Report.
European Medicines Agency. (2024). Orphan Medicinal Products: Regulatory Guidelines and Market Analysis. Amsterdam: EMA Publications.
National Institutes of Health. (2024). Rare Diseases Clinical Research Network: Scientific Advances and Patient Impact. NIH Publication No. 24-7892.

