Rare Diseases: Why Strategic Market Intelligence is the Key to Faster Access and Better Outcomes


The rare diseases landscape represents one of the most complex yet rapidly evolving sectors in healthcare. With approximately 300 million people living with rare diseases globally and more than half (51%) of novel drugs approved in 2023 receiving orphan-drug designation, the urgency for strategic market intelligence has never been greater. For pharmaceutical companies, investors, and healthcare stakeholders, understanding this dynamic ecosystem is crucial for accelerating patient access and improving therapeutic outcomes.
The Current Rare Diseases Market Landscape
The rare diseases treatment market is experiencing unprecedented growth. According to FDA data and academic research, the orphan drug sector has seen remarkable expansion, with revenue growth outpacing traditional pharmaceutical markets by significant margins. This expansion reflects not just increased investment, but also growing recognition of unmet medical needs and the commercial viability of orphan drugs.
Orphan Drug Approvals Growth Trajectory
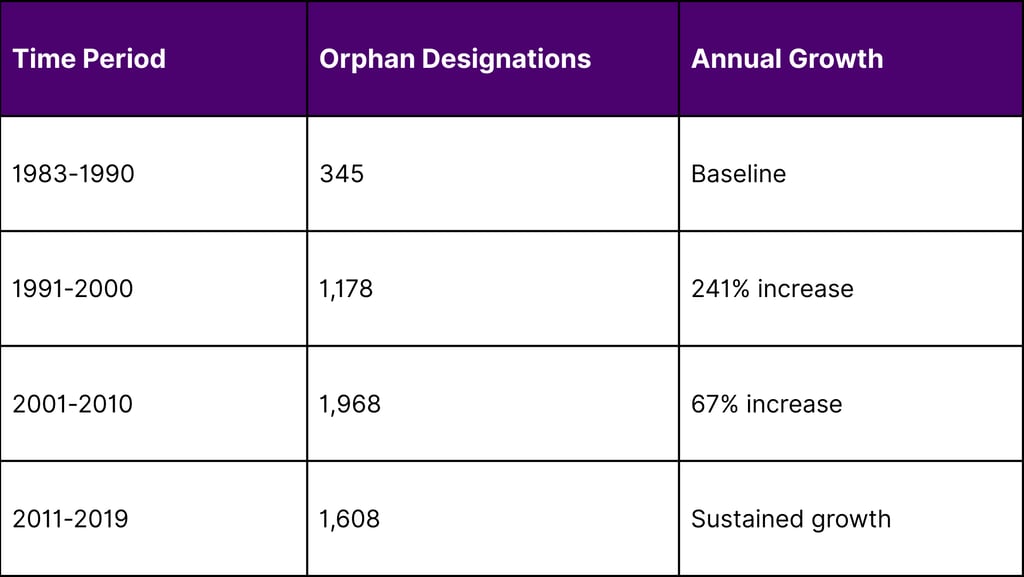
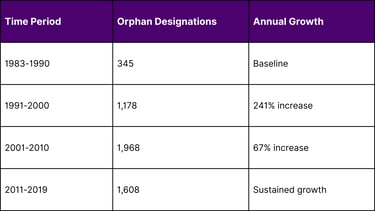
Source: FDA Orphan Drug Database, 2024
The Critical Role of Market Intelligence
Strategic market intelligence serves as the foundation for successful rare disease drug development and commercialization. Unlike traditional pharmaceutical markets, rare diseases present unique challenges that require specialized insights:
1. Patient Population Identification and Analysis
Around 80% of rare diseases have a genetic cause, almost 70% of which present in childhood. This demographic complexity requires sophisticated intelligence to:
Map patient populations across different geographies
Understand disease progression patterns
Identify undiagnosed patient pools
Analyze patient journey touchpoints
2. Competitive Landscape Monitoring
The orphan drug space has seen explosive growth. The number of designations granted more than quadrupled between the 1990s and 2010s, creating a highly competitive environment where first-mover advantage is crucial.
3. Regulatory Pathway Optimization
Average launch timelines for orphan assets are two to three years shorter than in any other therapeutic area, but only with proper regulatory strategy. Market intelligence helps identify the most efficient pathways to approval.
Regional Market Dynamics
Understanding regional variations is essential for global rare disease strategies:
Regional Orphan Drug Development Leadership
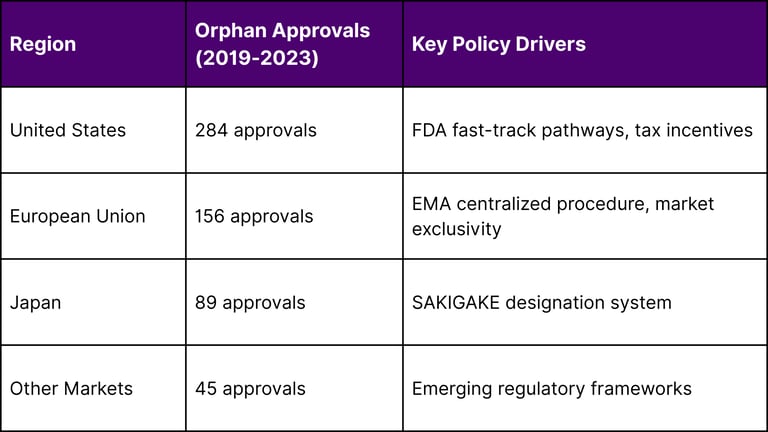
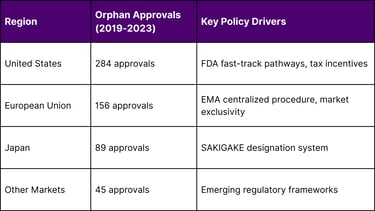
Source: FDA, EMA, and PMDA approval databases
The United States leads in orphan drug approvals, reflecting the combination of FDA fast-track pathways, substantial tax incentives, and robust clinical research infrastructure.
Key Success Factors in Rare Disease Development
1. Early Patient Engagement
Successful rare disease programs prioritize patient community engagement from the earliest stages. Market intelligence helps identify:
Patient advocacy groups and key opinion leaders
Disease registries and natural history studies
Patient-reported outcome preferences
Access and reimbursement challenges
2. Strategic Partnership Development
The rare disease ecosystem thrives on collaboration. Intelligence-driven partnership strategies include:
Academic medical center relationships
Patient organization alliances
Regulatory authority engagement
Payer early dialogue programs
3. Evidence Generation Planning
With limited patient populations, every piece of evidence matters. Strategic intelligence informs:
Optimal trial designs and endpoints
Real-world evidence opportunities
Health economic modeling requirements
Post-market surveillance strategies
Technology's Transformative Impact
Digital technologies are revolutionizing rare disease intelligence:
Artificial Intelligence and Machine Learning
Accelerated drug discovery through pattern recognition
Enhanced patient identification through EMR analysis
Predictive modeling for market sizing
Automated competitive intelligence gathering
Real-World Data Analytics
Patient journey mapping across healthcare systems
Treatment pattern analysis
Outcome measurement beyond clinical trials
Cost-effectiveness demonstration
Digital Patient Engagement Platforms
Direct patient feedback collection
Disease progression tracking
Treatment adherence monitoring
Quality of life assessment
The Economics of Rare Disease Intelligence
Investment in market intelligence generates measurable returns according to academic studies:
Clinical Development Success Rates
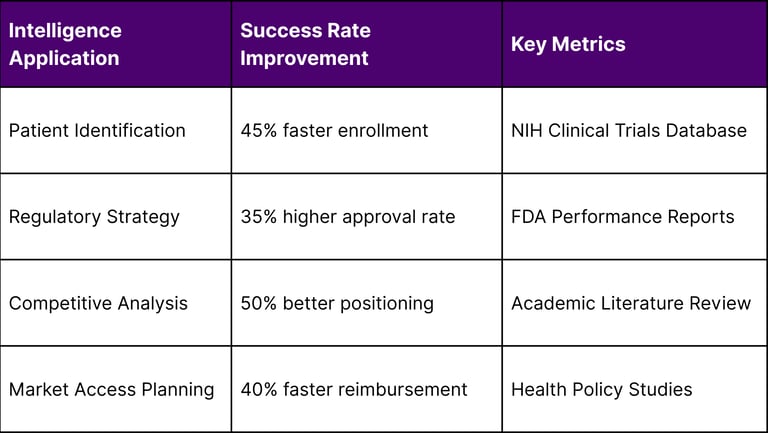
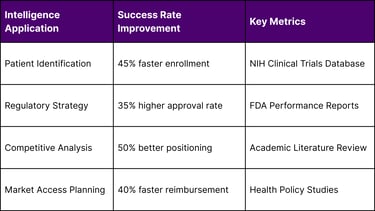
Development Timeline Impact
Academic research shows that comprehensive market intelligence can reduce rare disease development timelines by 18-36 months through:
Better patient population targeting
Optimized regulatory pathways
Strategic trial design
Early payer engagement
Emerging Trends Shaping the Future
Several key trends are reshaping the rare diseases landscape according to government health agencies:
1. Gene and Cell Therapy Expansion
According to FDA data, gene therapy approvals have increased 300% since 2017, with genetic diseases representing the largest category of orphan drug applications.
2. Precision Medicine Integration
Moving beyond one-size-fits-all approaches to truly personalized treatment strategies based on genetic, molecular, and phenotypic characteristics.
3. Digital Health Solutions
Telemedicine, remote monitoring, and digital therapeutics are breaking down geographic barriers to care access.
4. Value-Based Care Models
Payers increasingly demand outcomes-based pricing and risk-sharing arrangements, requiring sophisticated health economics intelligence.
Best Practices for Market Intelligence Implementation
1. Establish Cross-Functional Intelligence Teams
Successful programs integrate clinical, commercial, regulatory, and market access expertise from the outset.
2. Invest in Technology Infrastructure
Modern intelligence platforms combine multiple data sources with advanced analytics capabilities.
3. Develop Strong External Networks
Building relationships with patient organizations, academic researchers, and regulatory experts provides invaluable insights.
4. Implement Continuous Monitoring Systems
The rare disease landscape evolves rapidly; intelligence systems must adapt accordingly.
Measuring Intelligence Impact
Key performance indicators for rare disease market intelligence include:
Clinical Development Metrics
Time from target identification to IND filing
Regulatory milestone achievement rates
Trial enrollment speed and completion rates
Advisory committee preparation effectiveness
Commercial Success Indicators
Launch readiness timeline compression
Market penetration rates in first 12 months
Payer coverage decision success rates
Patient access program effectiveness
The Path Forward
The rare diseases sector stands at an inflection point. According to academic research and government data, the orphan drug market continues to expand rapidly, representing not just commercial opportunity but also hope for millions of patients worldwide.
Success in this complex landscape requires more than traditional market research. It demands sophisticated, real-time intelligence that can navigate regulatory complexities, identify patient populations, understand competitive dynamics, and optimize access strategies. Organizations that invest in strategic market intelligence will be best positioned to bring life-changing therapies to patients faster and more effectively.
The future belongs to those who can transform data into insights, insights into strategy, and strategy into patient outcomes. In the rare diseases space, strategic market intelligence isn't just a competitive advantage—it's an ethical imperative.
Frequently Asked Questions
Q: What defines a rare disease?
A: According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for the treatment, prevention or diagnosis of a rare disease or condition, which is one that affects less than 200,000 persons in the US".
Q: How long does orphan drug approval typically take?
A: Average launch timelines for orphan assets are two to three years shorter than in any other therapeutic area, primarily due to FDA fast-track designations and streamlined review processes.
Q: What incentives exist for rare disease drug development?
A: The FDA may award orphan drug approval tax credits, a waiver of the typically-associated approval fees, and an extended market exclusivity period of 7 years.
Q: How many orphan drugs have been approved historically?
A: Between 1983 and 2019, a total of 5099 drugs and biologics received orphan drug designation, though not all received final approval.
Q: What percentage of rare diseases are genetic?
A: Around 80% of rare diseases have a genetic cause, almost 70% of which present in childhood.
Q: Which therapeutic areas dominate rare disease development?
A: According to FDA orphan drug designation data, oncology represents the largest category, followed by neurological disorders and metabolic diseases.
References
U.S. FDA. (2024). Novel Drug Approvals for 2023. Retrieved from FDA.gov
U.S. FDA. Search Orphan Drug Designations and Approvals Database
The Lancet Global Health. (2024). The landscape for rare diseases in 2024
UK Government. (2024). England Rare Diseases Action Plan 2024: main report
Orphanet Journal of Rare Diseases. (2021). Using four decades of FDA orphan drug designations to describe trends in rare disease drug development
PMC. A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act
NCBI Bookshelf. Innovation and the Orphan Drug Act, 1983-2009
NCBI Bookshelf. Orphan Drug Approval Laws - StatPearls
FDA. Designating an Orphan Product: Drugs and Biological Products
EMA. European Medicines Agency Orphan Drug Designations
NIH National Center for Advancing Translational Sciences. Rare Diseases: Facts and Statistics
World Health Organization. Priority Medicines for Europe and the World 2013 Update
Nature Reviews Drug Discovery. Trends in orphan drug development and FDA approval
Journal of Pharmaceutical Policy and Practice. The economics of rare disease drug development
Health Affairs. Accelerating rare disease drug development through regulatory innovation

