Revolutionizing Cancer Treatment


Smart nanotherapies represent the convergence of nanotechnology and molecular oncology to revolutionize how cancer is diagnosed, targeted, and treated. Unlike traditional chemotherapies, these ultra-precise platforms leverage nanoscale materials—often less than 100 nanometers in size—to deliver drugs directly to tumor sites with enhanced selectivity and minimal side effects.
Asia has emerged as a global leader in the deployment and advancement of smart nanotherapies. Home to some of the most rapidly aging populations and diverse cancer profiles, countries like Japan, China, Singapore, and South Korea are investing heavily in nanotech-driven solutions to meet escalating healthcare demands. This article explores the transformative impact of smart nanotherapies on cancer treatment in Asia and the continent’s growing role as a powerhouse in global nanomedicine innovation.
Rise of Precision Oncology in Asia
The paradigm shift from one-size-fits-all chemotherapy to targeted, biomarker-driven therapies is well underway across Asia. Precision oncology integrates genomic profiling, artificial intelligence, and real-time diagnostics to enable hyper-personalized treatment regimens—and smart nanotherapies are a cornerstone of this transition.
Countries like China and Japan are integrating AI platforms with national cancer registries to drive patient stratification, while Singapore and South Korea have launched national genomic projects aimed at supporting personalized treatment models.
Key Drivers:
Expansion of genomic databases and AI-enhanced diagnostics
Increasing incidence of resistant cancers (e.g., triple-negative breast cancer)
Government subsidies for next-generation therapeutics
Smart Nanocarriers: The Backbone of Innovation
Engineered delivery vehicles known as nanocarriers are the foundation of smart nanotherapies. These structures are designed to overcome biological barriers and deliver therapeutic payloads selectively to tumors while sparing healthy tissue.
Common Nanocarrier Types:
Liposomes – Used in breast and liver cancers
Polymeric Micelles – Encapsulate hydrophobic drugs
Dendrimers – Branched polymers for multivalent loading
Exosomes – Natural vesicles for immune modulation
These leverage mechanisms like the Enhanced Permeability and Retention (EPR) effect, active targeting, and stimuli-responsive systems. Applications include lung, colorectal, liver, and pancreatic cancers.
Clinical Advancements and Research Highlights
Asia’s leading universities and medical research centers have delivered notable nanotherapy advancements:
Japan’s RIKEN developed photothermal nanotherapy for skin cancer.
South Korea’s CHA University is exploring biodegradable nanocarriers.
China’s NCNST advanced brain-penetrating liposomal therapies.
These achievements signify Asia’s growing clinical innovation in nanomedicine.
Leading Institutions and Government Initiatives
Prominent Institutions:
NUS (Singapore): Cancer nanomedicine development
Chinese Academy of Sciences: Regulatory science and nanoparticle safety
RIKEN (Japan): Cancer theranostics research
Government initiatives like China’s 13th Five-Year Plan and Japan’s Moonshot R&D program are strengthening Asia’s leadership in smart nanotherapies.
Asia’s Nanomedicine Startups and Biotech Ecosystem
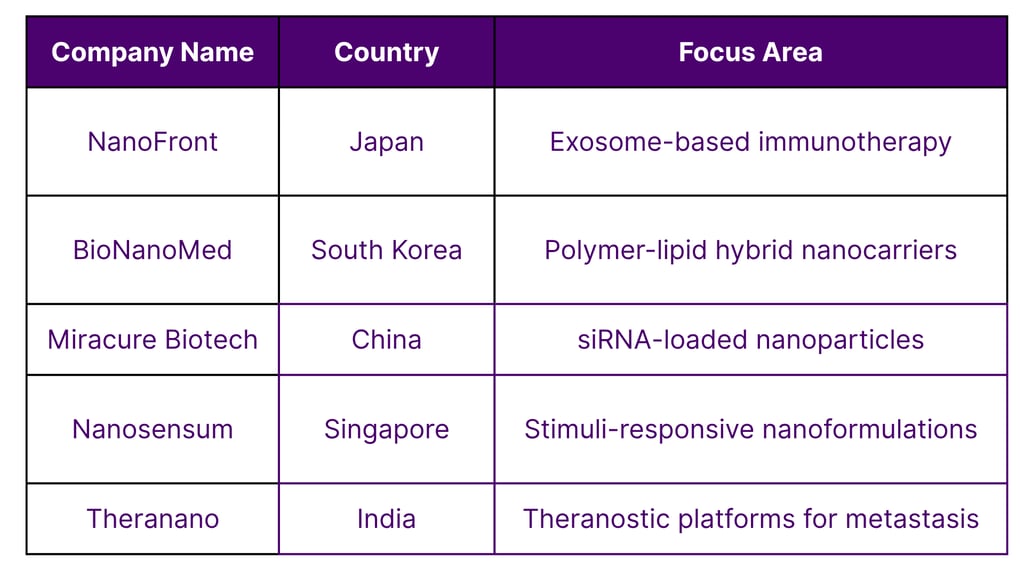
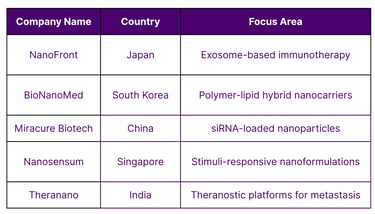
These startups demonstrate how innovation and IP development are thriving across Asia.
Oncology Nanotech Investment in Asia (2015–2025)
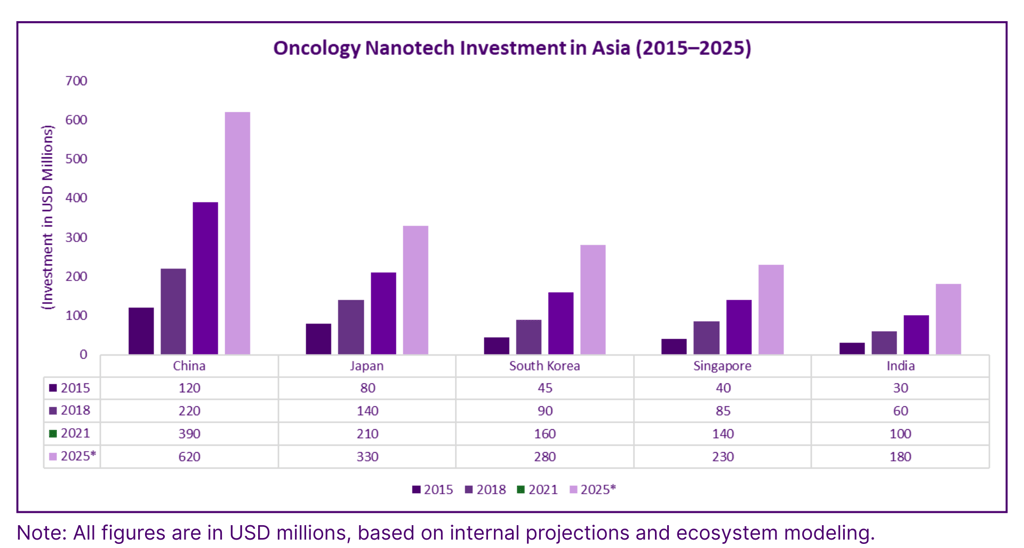
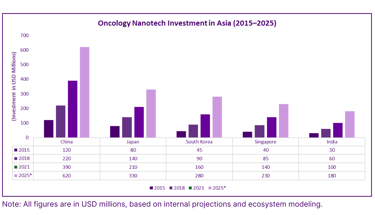
Manufacturing and Scalability of Nanotherapies
Scalable, GMP-compliant manufacturing is essential for clinical and commercial success. Singapore and China are emerging as key manufacturing centers with:
Nano-characterization labs
Sterile pilot plants
CDMO partnerships
Standardization and skilled workforce development remain focal areas.
Regulatory Pathways and Harmonization
Highlights:
China (NMPA): Guidelines for nanodrug evaluation
Singapore (HSA): Fast-track review schemes for nanotech
Japan (PMDA): Global harmonization with ICH
ASEAN and RCEP initiatives are fostering cross-border regulatory convergence for nanotherapies.
Addressing Equity and Access
Access remains unequal across Asia. Solutions include:
Government subsidies and reimbursement pilots
NGO-sponsored trials in rural zones
Integration with universal healthcare (e.g., Thailand, Malaysia)
Early pilot programs are now exploring how to list nanotherapies on national formularies.
Applications Beyond Oncology
Nanotech is now applied to:
Cardiology: Ischemia repair via nanoparticles
Neurology: Liposomal brain delivery
Autoimmune Disorders: Nano-immunosuppressants
Asia is becoming a test bed for expanding the utility of smart nanotherapies across diseases.
Personalized Nanomedicine: AI Meets Nanotech
AI is helping design and optimize smart nanotherapies by:
Predicting nanoparticle behavior
Tailoring formulations to patient genetics
Matching patients to trials using biomarkers
This convergence promises highly personalized and adaptive oncology treatments.
Challenges and Ethical Considerations
Key concerns include:
Nanotoxicology: Long-term safety studies still ongoing
Biodegradability: Ensuring safe breakdown and clearance
Ethical Communication: Complex informed consent for patients
Asian ethics committees are evolving to meet these unique nanomedicine challenges.
The Future: Smart Nanorobots and Theranostics
Asia is pioneering innovations like:
Japan: Magnetic-guided nanorobots
South Korea: Theranostic drug-diagnostics integration
Singapore: Organ-on-chip platforms for testing nanodevices
By 2030, clinical use of autonomous nanorobots could become a reality in oncology.
Conclusion
Asia is not only catching up—it’s leading the global nanomedicine race. With strong institutional support, advanced manufacturing capabilities, and a commitment to personalized care, smart nanotherapies are redefining the future of cancer treatment across the region.
FAQs
1. What are smart nanotherapies and how do they work in cancer treatment?
Smart nanotherapies deliver drugs directly to tumors using nanoscale carriers, improving effectiveness and minimizing side effects.
2. Why is Asia investing heavily in nanomedicine?
Rapidly growing cancer rates and innovation-friendly ecosystems make Asia a hotspot for nanotech-driven healthcare solutions.
3. Which cancers are most commonly treated using nanotherapies?
Liver, breast, lung, and colorectal cancers see the most applications due to tumor accessibility and research priorities.
4. Are smart nanotherapies accessible in lower-income Asian countries?
Access is improving through insurance integration, hospital partnerships, and government pilot schemes.
5. What’s the future of nanorobotics in cancer therapy?
Smart nanorobots may soon deliver autonomous treatments with real-time diagnostics, offering unprecedented precision.
References & Data Sources
Government funding documents (e.g., China’s 13th Five-Year Plan, RIE2025)
Academic research from NUS, RIKEN, NCNST, and A*STAR
Patent data and open-access clinical trials registries
Investment trend modeling based on regional biotech funding data

