Singapore’s Biomanufacturing Boom


Biomanufacturing has become one of the most critical components of modern pharmaceutical production, encompassing everything from vaccine development to advanced biologics and gene therapies. In this high-stakes global arena, Singapore has decisively transformed itself into a leading biomanufacturing hub. With a strategic location, pro-innovation policies, world-class infrastructure, and strong regulatory frameworks, Singapore is not just keeping pace with global leaders—it’s setting the standard. This article explores how Singapore achieved its pharmaceutical prominence and what lies ahead in its journey.
Strategic Government Vision and Policy Support
Singapore’s biomanufacturing success is rooted in deliberate, long-term government strategy. The country’s Biomedical Sciences Initiative, launched in 2000, laid the groundwork for a thriving ecosystem by investing heavily in infrastructure, talent, and translational research. The Research, Innovation and Enterprise 2025 (RIE2025) plan allocates over SGD 25 billion for R&D, with biopharma a central focus.
The Economic Development Board (EDB) has played a key role in attracting biopharma investment through policy incentives, infrastructure support, and talent development partnerships. Its efforts have helped position Singapore as a stable and innovation-driven environment for advanced pharmaceutical manufacturing.
Infrastructure for Innovation: Biopolis and Tuas Biomedical Park
Singapore’s purpose-built biomedical hubs—Biopolis and Tuas Biomedical Park—are critical to its success.
Biopolis hosts public research institutes, biotech startups, and multinational corporations in a collaborative ecosystem.
Tuas Biomedical Park supports large-scale production, offering pre-zoned land, utilities, and streamlined logistics for manufacturing operations.
These hubs enable seamless collaboration between R&D and commercial manufacturing activities.
Workforce Excellence and Talent Development
Singapore’s education and training ecosystem supports a continuous pipeline of high-caliber biotech professionals. World-class institutions such as NUS, NTU, and A*STAR focus on equipping graduates with skills in:
GMP-compliant production
Bioprocessing and automation
Digital analytics and robotics
Strong government-academia-industry partnerships ensure talent development aligned with industry needs.
Regulatory Strength and Global Compliance
Singapore’s Health Sciences Authority (HSA) maintains alignment with global regulatory norms, including ICH and GMP standards. Its transparent inspection and audit protocols enable:
Accelerated product approvals
Mutual recognition agreements with the US, EU, and Japan
Investor confidence in compliance and quality standards
Export Leadership in Biopharmaceuticals
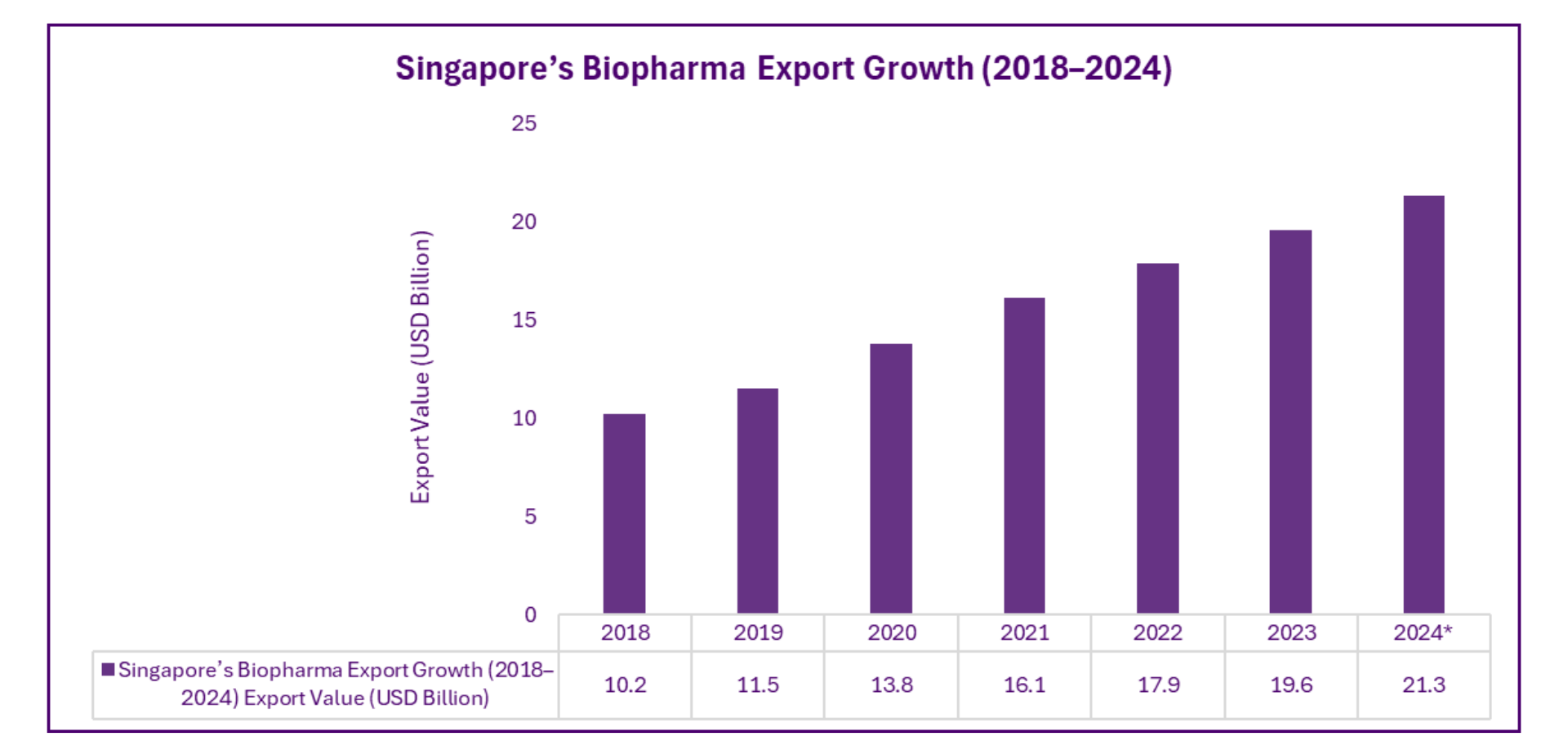
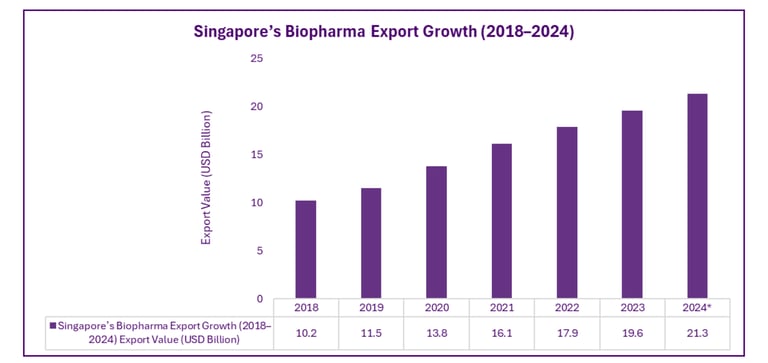
Singapore has established itself as a key node in global supply chains, with biopharma exports reaching major markets including the US, EU, and Asia-Pacific. The city-state’s logistics capabilities and IP protection frameworks make it a preferred base for pharmaceutical production and distribution.
Industry Growth and Investment Trends
Recent years have seen significant investment into Singapore’s biomanufacturing sector. Expansion initiatives have been supported by strategic investors and biopharma multinationals committed to building state-of-the-art facilities.
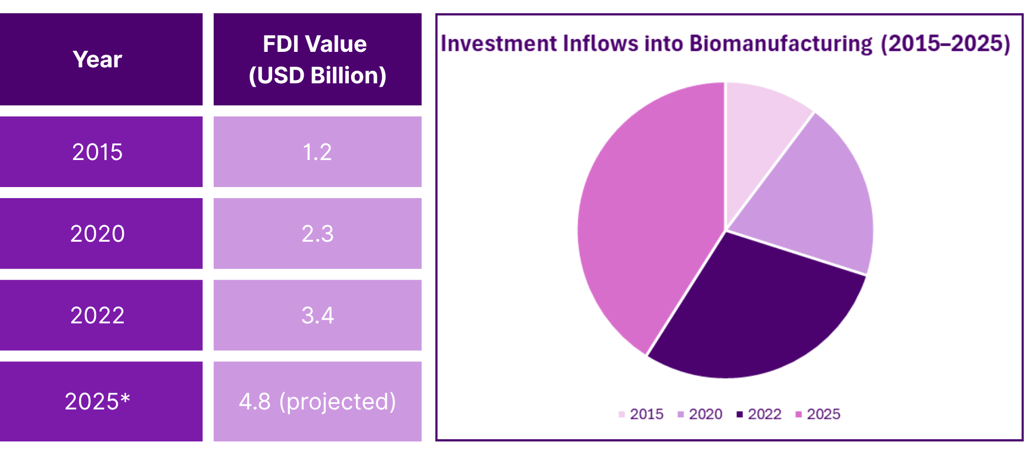
Projected Biomanufacturing Investment Growth (2015–2025):
Steady increase in capital inflows
Growth in biologics and advanced therapeutics capacity
New facility launches across industrial parks
Digitalization and Smart Manufacturing
Singapore is a leader in integrating Industry 4.0 practices into pharmaceutical manufacturing. These include:
IoT-enabled process monitoring
AI-driven quality control
Real-time analytics for batch consistency
Such digital transformation enhances productivity while reducing operational errors.
Sustainable Biomanufacturing Practices
Environmental sustainability is a core tenet of Singapore’s pharma strategy. Initiatives include:
Certified green buildings for biomanufacturing
Solar energy adoption and wastewater recycling
Emphasis on circular economy and green chemistry principles
These efforts support long-term resilience and environmental stewardship.
Vaccine Manufacturing and Pandemic Preparedness
Post-COVID, Singapore has scaled up vaccine manufacturing capabilities, including:
Local mRNA production capacity
Fill-finish platforms for multiple vaccine formats
Partnerships with international health alliances
These developments reinforce Singapore’s role in global health security planning.
Innovation Alliances and Global Partnerships
Singapore continues to build collaborative networks through:
R&D partnerships with leading global research institutions
Regional pharma and clinical trial collaborations
Harmonization efforts across regulatory bodies
These alliances accelerate innovation and market access.
IP, Patents, and Biotech Innovation
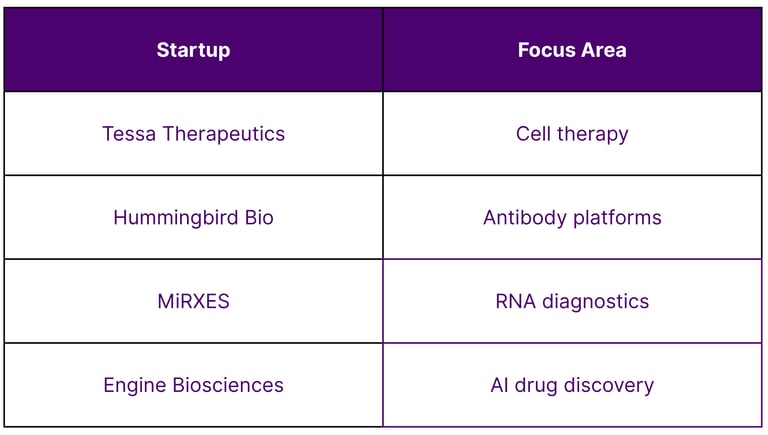
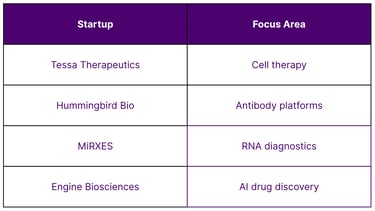
Singapore’s strong IP ecosystem supports commercialization of biotech innovation. Key contributors include:
Local biotech startups with proprietary platforms
Academic spin-offs in gene therapy and diagnostics
Patent-backed innovation across therapeutic verticals
Key Challenges and Strategic Responses
While Singapore’s success is clear, it must navigate challenges such as:
High cost of operations and real estate
Talent pipeline gaps in advanced roles
Supply chain fragility for certain APIs
Government and industry are working on targeted solutions to ensure competitiveness and sustainability.
The Future of Biomanufacturing in Singapore
Looking ahead to 2030, Singapore aims to:
Lead in cell and gene therapies
Integrate AI into end-to-end bioproduction
Anchor Asia's regulatory science and QA frameworks
These ambitions reflect a long-term vision of strategic global leadership.
Conclusion
Singapore’s pharmaceutical rise has been strategically engineered through thoughtful policy, infrastructure, and innovation. As a biomanufacturing hub, it offers agility, quality, and trust. For the global pharma industry, Singapore is not just an operational base—it’s a strategic imperative.
FAQs
1. Why is Singapore ideal for biomanufacturing?
Its regulatory strength, skilled workforce, and innovation infrastructure support end-to-end pharmaceutical excellence.
2. What are Singapore’s pharma priorities by 2030?
Advancing personalized medicine, scaling gene therapy, and integrating AI in pharma operations.
3. Which companies have operations in Singapore?
Major international and regional firms, with diverse manufacturing and research capabilities.
4. How does Singapore ensure sustainability in pharma?
Green certifications, resource-efficient technologies, and sustainability-focused industrial planning.
5. What measures support supply chain resilience?
Diversified sourcing strategies, digital supply tracking, and policy-driven stockpiling initiatives.
References & Data Sources
Singapore EDB (2024) – National Life Sciences and Biomanufacturing Strategies
HSA (2024) – Regulatory Guidelines and Compliance
RIE2025 Plan (2021) – Singapore Research & Innovation Priorities
A*STAR (2024) – National Biotech and Skills Development Programs
Internal Industry Analysis and Investment Modeling (2024)

