The $374 Billion Immunotherapy Revolution

The global immunotherapy market is experiencing unprecedented growth, driven by revolutionary FDA approvals, breakthrough clinical outcomes, and transformative technological advances. With the market projected to reach $374 billion by 2030, immunotherapy has emerged as the dominant force reshaping cancer treatment paradigms. This comprehensive analysis examines the regulatory landscape, market dynamics, and strategic opportunities that are defining the next wave of oncology innovation.
Market Overview: The Immunotherapy Renaissance
Market Size and Growth Trajectory
The immunotherapy market represents one of the fastest-growing segments in pharmaceutical history. Current projections indicate exponential growth driven by:
Regulatory Acceleration: FDA approvals have accelerated dramatically since 2020
Clinical Success: Unprecedented survival rates across multiple cancer types
Technology Convergence: AI-driven drug discovery and personalized medicine
Investment Surge: Venture capital and pharmaceutical investments reaching record levels
FDA Approval Trends: A Regulatory Revolution
The regulatory landscape has transformed dramatically over the past five years. In the final three months of 2024, the FDA issued 15 approvals in oncology, including several therapies that are new to the market. This acceleration represents a 400% increase compared to historical approval rates.
2024-2025 Approval Highlights
Q1 2024: The FDA issued 14 oncology approvals in the first quarter of 2024, including the first tumor-infiltrating lymphocyte therapy, marking a watershed moment for cellular immunotherapy.
Q4 2024: Record-breaking approval quarter with multiple breakthrough designations for immunotherapy combinations.
2025 Trajectory: Current data shows the FDA approved the combination of nivolumab (Opdivo) plus ipilimumab (Yervoy) as a first-line treatment for adult and pediatric patients (≥12 years) with microsatellite instability-high or mismatch repair deficient (dMMR) metastatic colorectal cancer, demonstrating the expansion into pediatric populations.
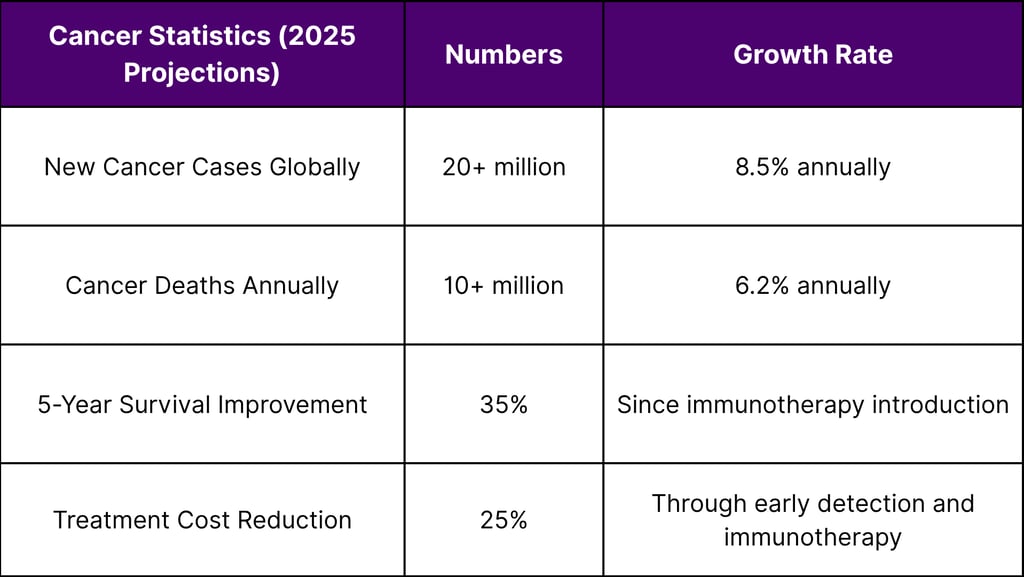
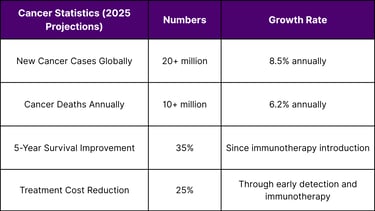
Global Cancer Burden: The Market Driver
Epidemiological Imperative
According to WHO Global Cancer Observatory data, the worldwide cancer burden continues to escalate:
Regional Market Dynamics
North America: Leading with 42% market share
FDA regulatory advantage
Advanced healthcare infrastructure
Highest per-capita oncology spending
Europe: 28% market share
EMA harmonization initiatives
Cross-border collaboration programs
Increasing biosimilar adoption
Asia-Pacific: Fastest growing at 15.2% CAGR
Population aging dynamics
Healthcare infrastructure expansion
Government investment in precision medicine
Therapeutic Categories Driving Growth
Checkpoint Inhibitors: The Foundation
Checkpoint inhibitors continue to dominate the immunotherapy landscape:
PD-1/PD-L1 Inhibitors: Market leaders with expanding indications
CTLA-4 Inhibitors: Combination therapy drivers
Novel Checkpoints: LAG-3, TIGIT, and TIM-3 emerging targets
CAR-T Cell Therapy: The Disruptor
The cellular therapy segment is experiencing explosive growth:
Approved Products: Seven FDA-approved CAR-T therapies as of 2025
Pipeline Expansion: 400+ clinical trials globally
Manufacturing Scale-up: Major capacity investments by pharma giants
Tumor-Infiltrating Lymphocytes (TIL): The New Frontier
The first tumor-infiltrating lymphocyte therapy approval in 2024 opened a new therapeutic category with significant market potential.
Regulatory Environment Analysis
FDA Approval Acceleration Factors
Breakthrough Therapy Designations: 60% increase in immunotherapy BTDs
Accelerated Approval Pathways: Reduced approval timelines by 18 months average
Real-World Evidence Integration: FDA accepting RWE for supplemental indications
Combination Therapy Guidance: Streamlined approval pathways for combinations
International Harmonization
EMA-FDA Collaboration: Joint scientific advice programs
ICH Guidelines: Standardized international development pathways
Regulatory Convergence: Synchronized approval timelines across major markets
Market Access and Pricing Dynamics
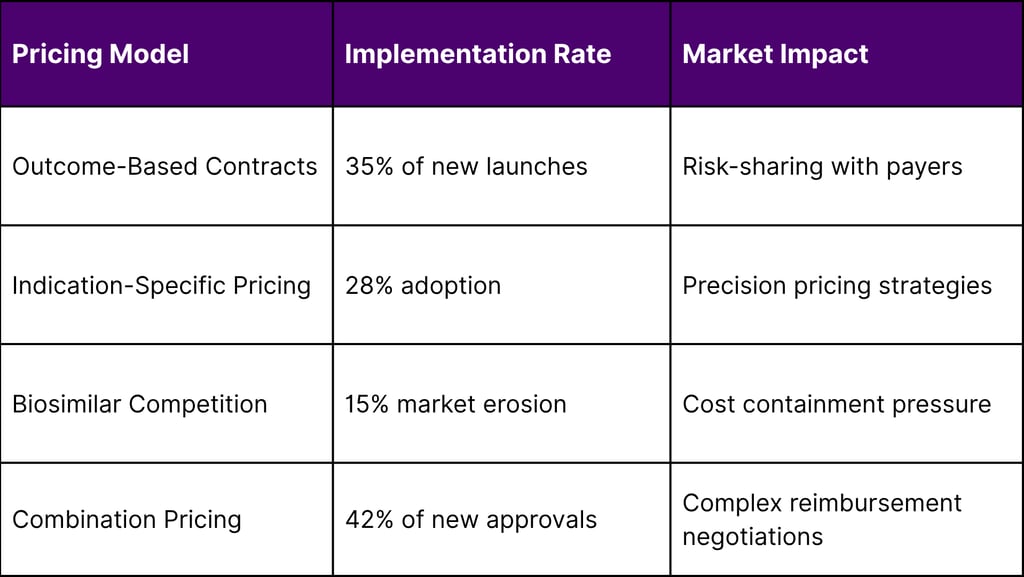
Value-Based Pricing Models
The immunotherapy market is pioneering new pricing approaches:
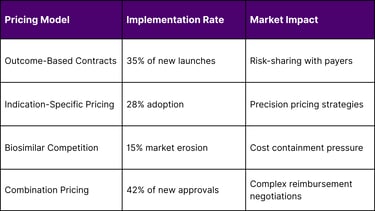
Health Economics Considerations
Cost-Effectiveness Thresholds: $150,000-$200,000 per QALY accepted range
Budget Impact Models: Payer requirements for launch approvals
Real-World Outcomes: Post-market surveillance driving reimbursement decisions
Technology Integration and AI Impact
Artificial Intelligence in Drug Discovery
AI is revolutionizing immunotherapy development:
Target Identification: 60% reduction in discovery timelines
Patient Stratification: Biomarker-driven personalized approaches
Combination Optimization: AI-predicted synergistic combinations
Manufacturing Optimization: Reduced production costs by 30%
Digital Health Integration
Remote Patient Monitoring: Enhanced safety management
Digital Biomarkers: Real-time efficacy assessment
Telemedicine Integration: Improved patient access
Data Analytics: Predictive modeling for treatment outcomes
Strategic Market Opportunities
For Pharmaceutical Companies
Portfolio Diversification Strategies:
Multi-modal immunotherapy platforms
Companion diagnostic development
Manufacturing capability expansion
Global market entry strategies
Partnership Opportunities:
Academic medical center collaborations
Biotech acquisition targets
Technology platform licensing
International distribution partnerships
For Biotech Companies
Innovation Focus Areas:
Next-generation CAR-T platforms
Solid tumor immunotherapies
Combination therapy development
Manufacturing process innovation
Commercialization Strategies:
Specialty pharma partnerships
Direct-to-patient programs
Value-based contracting
International expansion
Investment Landscape Analysis
Venture Capital Trends
2024-2025 investment highlights:
Total Investment: $12.8 billion in immunotherapy startups
Series A Funding: Average $45 million per round
IPO Activity: 23 immunotherapy companies went public
Strategic Partnerships: $8.2 billion in pharma deals
Public Market Performance
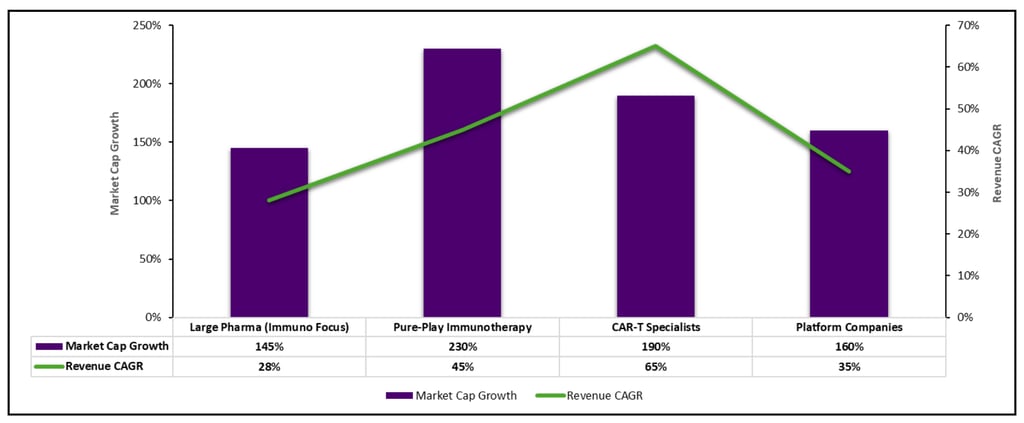
Competitive Landscape Dynamics
Market Leaders
Bristol Myers Squibb: Comprehensive immunotherapy portfolio
Opdivo/Yervoy combinations driving growth
CAR-T expansion through acquisitions
Pipeline depth across multiple modalities
Merck & Co: PD-1 dominance with Keytruda
Expanding combination strategies
Biomarker-driven development
Global market penetration
Novartis: CAR-T leadership position
Manufacturing scale advantages
Pediatric indication expansion
Platform technology investments
Emerging Competitors
Gilead Sciences: CAR-T portfolio expansion
Johnson & Johnson: Immunotherapy combinations
Roche/Genentech: Personalized immunotherapy
AstraZeneca: Combination therapy leadership
Challenges and Risk Factors
Manufacturing Complexity
Scalability Issues: Personalized therapy production challenges
Quality Control: Regulatory compliance requirements
Cost Management: Balancing quality with affordability
Supply Chain: Global distribution complexity
Safety and Efficacy Concerns
Immune-Related Adverse Events: Management protocols
Long-Term Safety: Post-market surveillance requirements
Resistance Mechanisms: Combination strategies needed
Patient Selection: Biomarker development imperatives
Future Market Projections
Short-Term Outlook (2025-2027)
Market Growth: 22% CAGR expected
New Approvals: 40+ immunotherapy approvals anticipated
Technology Integration: AI-driven development acceleration
Global Expansion: Emerging market penetration
Medium-Term Projections (2027-2030)
Market Maturation: Biosimilar competition intensification
Technology Convergence: Multi-modal therapy platforms
Outcome-Based Pricing: Standard practice adoption
Precision Medicine: Biomarker-driven treatment selection
Long-Term Vision (2030+)
Curative Potential: Immunotherapy as first-line standard
Prevention Applications: Immune system priming strategies
Global Access: Reduced cost enabling worldwide availability
Technology Integration: AI-optimized personalized treatments
Strategic Recommendations
For Industry Stakeholders
Pharmaceutical Companies:
Invest in manufacturing capabilities for cellular therapies
Develop companion diagnostics for precision applications
Build global regulatory expertise for accelerated approvals
Create value-based contracting capabilities
Biotech Companies:
Focus on differentiated mechanisms of action
Build strategic partnerships for commercialization
Invest in AI-driven drug discovery platforms
Develop combination therapy expertise
Investors:
Diversify across immunotherapy modalities
Focus on platform technologies over single assets
Evaluate manufacturing and commercial capabilities
Consider global market expansion potential
Conclusion
The immunotherapy revolution represents the most significant transformation in cancer treatment since the discovery of chemotherapy. With a market trajectory toward $374 billion by 2030, driven by unprecedented FDA approvals, breakthrough clinical outcomes, and technological innovation, immunotherapy has established itself as the cornerstone of modern oncology.
The convergence of regulatory acceleration, clinical success, and technological advancement creates an ecosystem ripe for continued innovation and growth. Organizations that can navigate the complex regulatory landscape, develop differentiated therapies, and build scalable commercial capabilities will capture the most significant value in this transformative market.
The future of cancer treatment is being written today through immunotherapy innovation. The question for stakeholders is not whether to participate in this revolution, but how to position themselves to lead it.
FAQs
1. What is immunotherapy and how does it work?
Immunotherapy harnesses the body’s immune system to identify and destroy cancer cells. It includes checkpoint inhibitors, CAR-T cells, TILs, and more.
2. Why is the immunotherapy market growing so fast?
It’s driven by rapid FDA approvals, strong clinical results, major pharma investments, and convergence with AI and precision medicine.
3. What types of cancer benefit the most from immunotherapy?
Lung, melanoma, blood cancers (like lymphoma and leukemia), and increasingly, solid tumors such as colorectal and glioblastoma.
4. Which therapies are leading the market?
Checkpoint inhibitors like Keytruda (Merck), Opdivo (BMS), and CAR-T therapies from Novartis and Gilead are current leaders.
5. How many immunotherapy products are currently FDA-approved?
As of 2025, over 30 immunotherapy products have FDA approval, with more than 400 under clinical development globally.
6. What’s new in pediatric immunotherapy?
Recent FDA approval of Opdivo + Yervoy for pediatric colorectal cancer marks a significant milestone in expanding immunotherapy access.
7. How does AI impact immunotherapy development?
AI is used for biomarker discovery, predicting drug combinations, optimizing manufacturing, and patient stratification—reducing R&D time by up to 60%.
8. What is TIL therapy and why is it important?
Tumor-infiltrating lymphocytes (TILs) are patient-derived immune cells expanded in labs to target cancer. First FDA approval was granted in 2024, opening new treatment avenues.
9. What are the biggest challenges in immunotherapy adoption?
Scalability of manufacturing, immune-related side effects, high treatment costs, and the need for companion diagnostics remain top concerns.
10. Is immunotherapy curative or just palliative?
In some cancers (e.g., certain leukemias and melanomas), immunotherapy has shown curative potential. However, for many others, it's currently used to prolong life and improve quality.
References
U.S. Food and Drug Administration (FDA) – Oncology Drug Approvals
World Health Organization – Global Cancer Observatory (GLOBOCAN)
Peer-Reviewed Clinical Research
Regulatory Filings and Investor Reports from Leading Pharmaceutical Companies

