The KRAS Inhibitor Race Heats Up


The landscape of KRAS-targeted therapy has undergone a remarkable transformation in 2025, shifting from a once "undruggable" target to a rapidly evolving therapeutic frontier. With approximately 250,000 new KRAS mutant cancer cases diagnosed annually in the United States alone, representing roughly 19% of all cancer patients, the clinical and commercial implications of recent breakthroughs are profound. This article examines the current state of KRAS inhibitor development, focusing on FDA-approved therapies, emerging G12D inhibitors entering late-stage trials, and the pivotal role of combination immunotherapy strategies that promise to reshape solid tumor treatment paradigms.
The KRAS Mutation Landscape: Understanding the Target
Prevalence Across Cancer Types
KRAS mutations represent one of the most common oncogenic drivers across multiple solid tumor types. According to data from the National Cancer Institute's RAS Initiative, approximately one third of all human cancers harbor mutations in the RAS gene family (KRAS, NRAS, and HRAS).
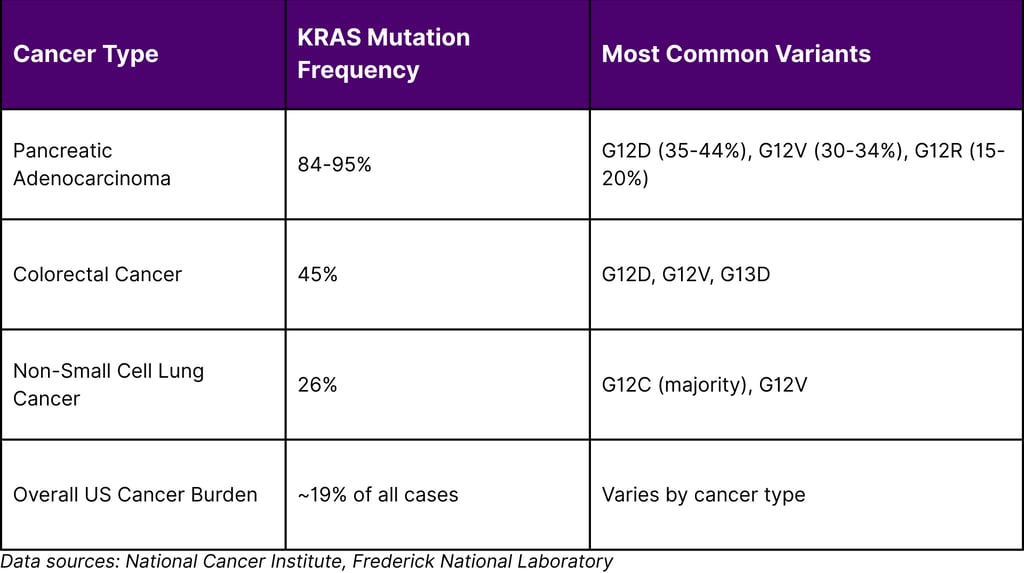
Table 1: KRAS Mutation Prevalence in Major Cancer Types

Mutation-Specific Characteristics
Different KRAS mutations show distinct prevalence patterns across cancer types. For instance, G12D mutations are particularly common in pancreatic cancers, whereas G12C mutations predominate in lung cancers. In pancreatic cancer specifically, the distribution shows KRAS G12D in approximately 35% of patients, G12V in 30%, and G12R in 15%.
Recent research from Memorial Sloan Kettering has revealed that these variants aren't merely interchangeable they demonstrate distinct biological behaviors. KRAS G12D has been associated with aggressive cancer and worse outcomes, while G12R and G12V correlate with better overall survival. These findings underscore the importance of variant-specific therapeutic approaches.
FDA-Approved KRAS Inhibitors: The Foundation
Sotorasib (Lumakras): The First Breakthrough
On May 28, 2021, the FDA granted accelerated approval to sotorasib for adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer. This approval marked a watershed moment, transforming KRAS from an "undruggable" target into a clinically validated therapeutic opportunity.
Clinical Performance:
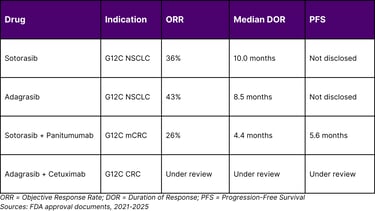
Objective response rate: 36% with median response duration of 10 months
Recommended dose: 960 mg orally once daily
Most common adverse reactions: diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity
2025 Expansion: On January 16, 2025, the FDA approved sotorasib with panitumumab for adult patients with KRAS G12C-mutated metastatic colorectal cancer. In the CodeBreaK 300 trial, median progression-free survival was 5.6 months in the sotorasib/panitumumab arm compared to 2 months in the standard of care arm.
Adagrasib (Krazati): Expanding the Arsenal
On December 12, 2022, the FDA granted accelerated approval to adagrasib for KRAS G12C-mutated locally advanced or metastatic NSCLC. The KRYSTAL-1 trial demonstrated compelling efficacy, with an overall response rate of 43% and median duration of response of 8.5 months.
Key Development in 2024: On June 21, 2024, the FDA granted accelerated approval to adagrasib plus cetuximab for adults with KRAS G12C-mutated locally advanced or metastatic colorectal cancer. The recommended adagrasib dose is 600 mg orally twice daily until disease progression or unacceptable toxicity.
The G12D Revolution: Next-Generation Inhibitors
MRTX1133: Targeting the Most Common Pancreatic Cancer Mutation
While G12C inhibitors have achieved clinical success in lung cancer, the KRAS G12D mutation is present in more than one in three pancreatic cancers and about one in ten colorectal cancers. G12D is the most common KRAS mutation and is estimated to occur in more than 50,000 new cases of cancer in the United States each year.
MRTX1133, developed by Mirati Therapeutics, represents the first KRAS G12D-selective inhibitor to enter clinical trials. According to NCI research published in Cancer Discovery, MRTX1133 shrank tumors or halted their growth in several mouse models of human pancreatic cancer with KRAS G12D mutations, including the rigorous KPC mouse model that closely mimics human disease.
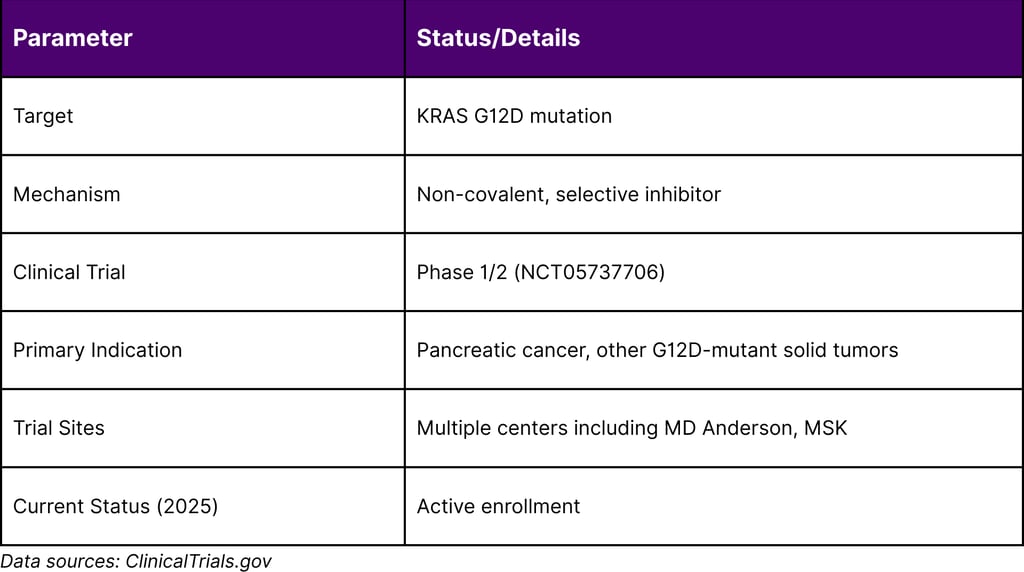
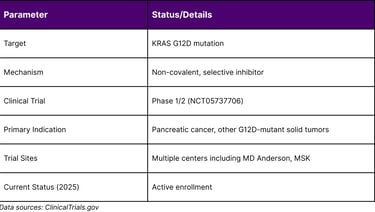
Table 2: MRTX1133 Clinical Development Status
Preclinical Insights and Combination Strategies
Research from MD Anderson Cancer Center published in 2023 has been particularly illuminating regarding MRTX1133's mechanism and optimal deployment. The studies revealed that while MRTX1133 monotherapy showed initial promise, tumors eventually developed resistance. However, combining MRTX1133 with immune checkpoint inhibitors led to sustained tumor regression and significantly improved survival in preclinical models.
These findings have directly informed clinical trial design. A Phase I clinical trial is currently ongoing at MD Anderson and other centers, incorporating combination strategies based on these preclinical insights.
Immunotherapy Combinations: The Critical Synergy
Why Monotherapy Isn't Enough
One of the most significant insights from 2025 research is that KRAS inhibition alone, while effective initially, often leads to tumor recurrence. Studies in pancreatic cancer models demonstrated that KRAS inhibition activates the Fas pathway and increases CD8+ T cell infiltration, but sustained responses require active immune participation.
When KRAS inhibitors are combined with checkpoint inhibitors, there's a reduced likelihood of resistance because these drugs work through completely different mechanisms. This dual-mechanism approach simultaneously attacks the cancer through oncogene inhibition and immune activation.
Clinical Trials in Progress
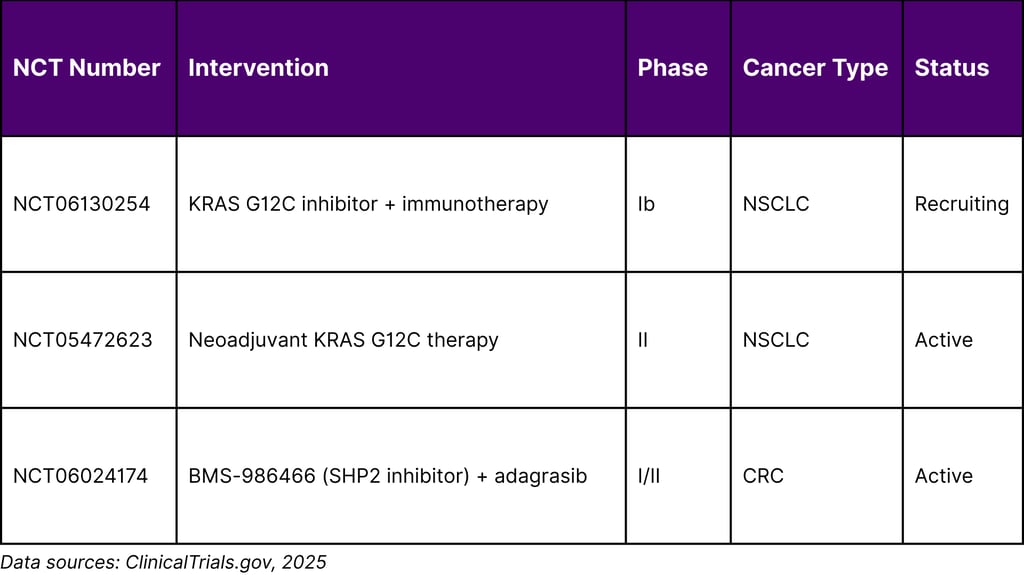
Multiple clinical trials are evaluating KRAS inhibitor-immunotherapy combinations:
Table 3: Select KRAS Inhibitor + Immunotherapy Combination Trials
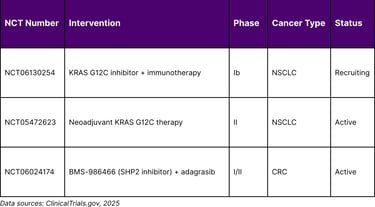
Emerging Data: KRAS G12C Inhibitors in Pancreatic Cancer
While G12C mutations occur in only 1-2% of pancreatic cancers, early trial data has been encouraging. In a trial involving 38 patients with advanced pancreatic cancer, sotorasib shrank tumors in about 20% of participants. Similar results were observed with adagrasib in colorectal cancer trials.
Market Dynamics and Pipeline Analysis
The Commercial Opportunity
With approximately 250,000 KRAS-mutant cancer diagnoses annually in the US, and millions globally, the commercial potential for effective KRAS inhibitors is substantial. The market is currently segmented by:
G12C inhibitors (established, growing indications)
G12D inhibitors (emerging, largest addressable market)
Pan-KRAS inhibitors (early development)
Combination therapies (future standard of care)
Competitive Landscape 2025
KRAS Inhibitor Development Timeline
2021: Sotorasib FDA approval (G12C, NSCLC)
2022: Adagrasib FDA approval (G12C, NSCLC)
2024: Combination approvals (G12C + EGFR inhibitors, CRC)
2025: MRTX1133 Phase 1/2 (G12D, pancreatic cancer); Multiple combination trials initiated; Pan-RAS inhibitors entering Phase 1
Pipeline Depth by Variant
Table 4: KRAS Inhibitor Pipeline by Mutation Target
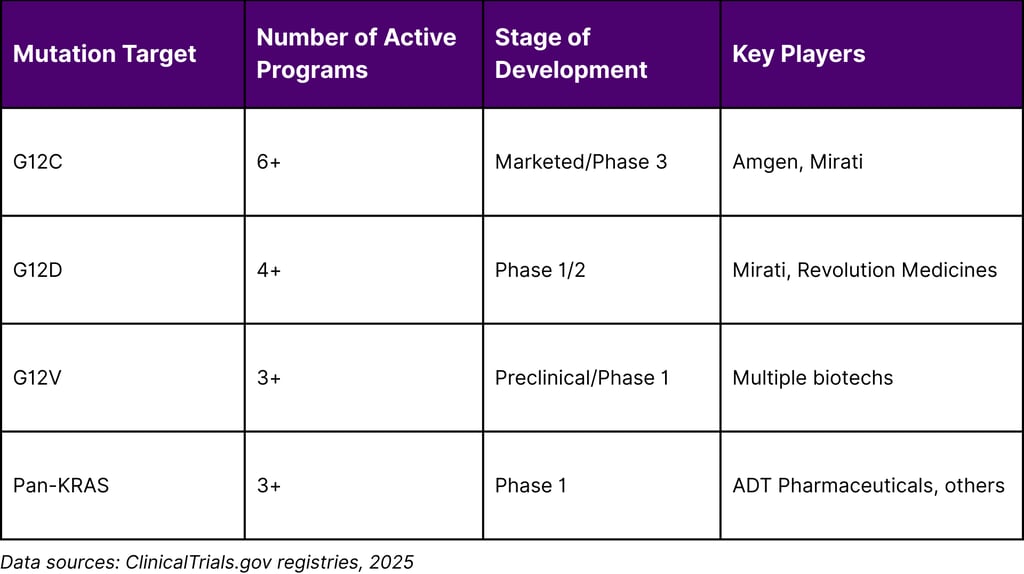
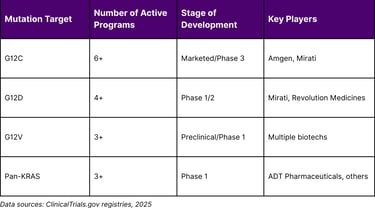
Clinical Implications and Treatment Paradigms
Biomarker Testing Becomes Critical
The variant-specific nature of KRAS inhibitors makes comprehensive molecular profiling essential. Current recommendations include:
Tissue-based NGS for all advanced NSCLC, CRC, and pancreatic cancer
Liquid biopsy for monitoring and resistance detection
Variant-specific reporting (not just "KRAS mutant" but specific codon)
Sequencing Strategies
The optimal sequencing of KRAS inhibitors with other therapies remains under investigation:
For NSCLC with G12C:
Platinum-based chemotherapy + immunotherapy → KRAS inhibitor at progression
Or: KRAS inhibitor + immunotherapy (under investigation)
For Pancreatic Cancer with G12D:
Standard chemotherapy (FOLFIRINOX/Gem-Abraxane) → KRAS inhibitor + immunotherapy (clinical trial)
Neoadjuvant KRAS inhibitor strategies under investigation
Resistance Mechanisms and Future Directions
Understanding Resistance
Multiple resistance mechanisms to KRAS G12C inhibitors have been identified:
Secondary KRAS mutations (G12D/R/V/W, G13D, Q61H, H95D/Q/R, Y96C)
Bypass pathway activation (EGFR, NRAS, BRAF amplification)
Immune escape mechanisms
Tumor microenvironment factors (particularly in pancreatic cancer)
Next-Generation Strategies
Pan-KRAS Inhibitors: Several programs are developing inhibitors that can target multiple KRAS variants simultaneously, potentially addressing both primary resistance and acquired mutations.
PROTACs and Protein Degraders: Proteolysis-targeting chimera (PROTAC) technology offers a novel approach by degrading mutant KRAS protein rather than merely inhibiting its function.
Metabolic Targeting: KRAS mutations reprogram cancer metabolism, creating vulnerabilities in glycolysis, macropinocytosis, and autophagy that can be therapeutically exploited.
Statistical Snapshot: KRAS Inhibitor Performance
Comparative Efficacy Across KRAS Inhibitors
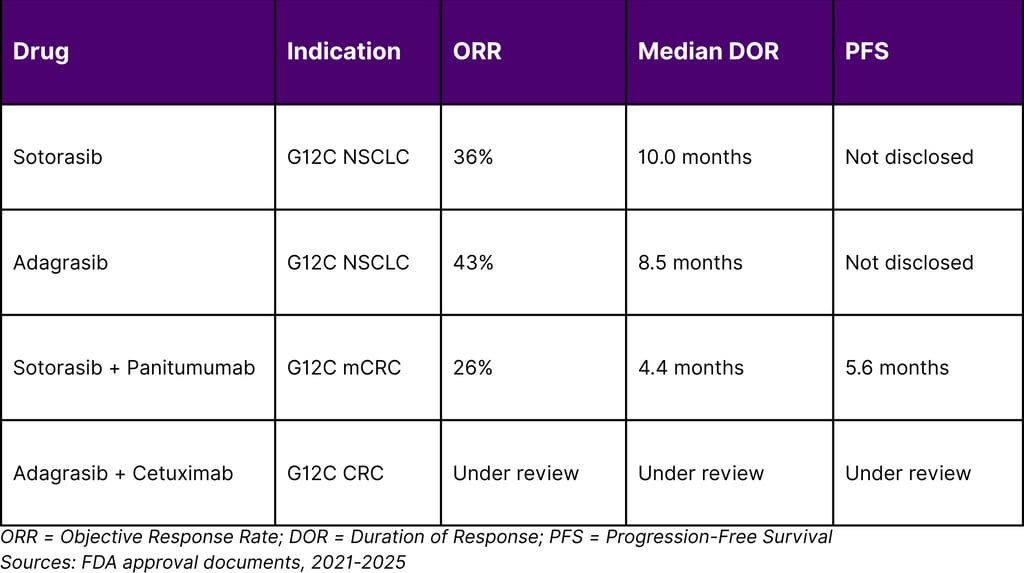
Key Takeaways for Stakeholders
For Oncology Strategists
KRAS is no longer undruggable – Multiple validated targets with approved therapies
Variant matters – G12C, G12D, and other variants require different therapeutic approaches
Combinations are critical – Monotherapy shows promise but combinations with immunotherapy or targeted agents appear superior
Pancreatic cancer breakthrough potential – G12D inhibitors could transform treatment of this historically refractory disease
For Investors and Business Development
Market expansion – From initial NSCLC approval to multiple tumor types by 2025
Pipeline depth – 15+ programs across multiple variants in clinical development
Combination economics – Future standard of care likely involves multi-drug regimens
Diagnostic co-development – Companion diagnostics for variant identification create parallel opportunities
For Clinical Trial Planners
Biomarker-driven enrollment – Precise variant identification essential
Adaptive designs – Flexibility to pursue promising combinations
TME considerations – Tumor microenvironment profoundly affects response, especially in pancreatic cancer
Patient selection – Prior therapy lines and co-mutations influence outcomes
Conclusion
The KRAS inhibitor landscape in 2025 represents a remarkable evolution from therapeutic futility to clinical reality. With G12C inhibitors now approved across multiple indications, G12D inhibitors entering pivotal trials, and combination immunotherapy strategies showing promise, the field is poised for continued rapid advancement.
For the approximately 250,000 patients annually diagnosed with KRAS-mutant cancers in the United States, these developments offer genuine hope. The variant-specific nature of these therapies underscores the importance of comprehensive molecular profiling and precision medicine approaches.
Looking forward, several key trends will shape the next phase of KRAS-targeted therapy development:
Expansion to earlier disease stages – Neoadjuvant and adjuvant trials are underway
Rational combination strategies – Pairing KRAS inhibitors with immunotherapy, chemotherapy, or other targeted agents
Resistance management – Development of next-generation inhibitors and pan-KRAS approaches
Biomarker refinement – Identifying which patients will benefit most from specific approaches
For oncology strategists, investors, and competitive intelligence teams, the KRAS inhibitor space represents one of the most dynamic areas in solid tumor drug development. The lessons learned from the G12C experience are now being applied to other variants, potentially unlocking effective therapies for historically intractable diseases like pancreatic cancer.
The race to develop better KRAS inhibitors continues to accelerate, and 2025 has proven to be a pivotal year in this ongoing story. As clinical trial data matures and regulatory approvals expand, KRAS-targeted therapy is positioned to become a cornerstone of precision oncology in the coming years.
Frequently Asked Questions
Q1: What makes KRAS such an important target in oncology?
KRAS mutations drive approximately 19% of all cancers in the United States, affecting over 250,000 new patients annually. It is the most commonly mutated oncogene across multiple solid tumor types, including pancreatic (84-95%), colorectal (45%), and lung (26%) cancers. For decades, KRAS was considered "undruggable" due to its structure, making recent therapeutic successes particularly significant.
Q2: Why are there different KRAS inhibitors for different mutations?
KRAS mutations at different codons (G12C, G12D, G12V, etc.) create structurally distinct proteins that require specifically designed inhibitors. G12C creates a unique cysteine residue that allows covalent inhibitor binding, while G12D requires a different binding approach. This variant-specificity is why multiple drug development programs are necessary to address the full spectrum of KRAS-mutant cancers.
Q3: What is the current FDA approval status of KRAS inhibitors?
As of 2025, the FDA has approved:
Sotorasib (Lumakras) for KRAS G12C-mutated NSCLC (2021) and with panitumumab for metastatic CRC (2025)
Adagrasib (Krazati) for KRAS G12C-mutated NSCLC (2022) and with cetuximab for CRC (2024)
Multiple additional programs, including MRTX1133 for G12D mutations, are in active clinical development.
Q4: Why is combination with immunotherapy important?
Preclinical and early clinical data suggest that KRAS inhibition alone often leads to initial responses followed by resistance. KRAS inhibition can activate immune pathways and increase T cell infiltration into tumors, creating a window for immunotherapy to work more effectively. Combining these approaches addresses both the oncogenic driver and the immune microenvironment, potentially leading to more durable responses.
Q5: What is the significance of MRTX1133 for pancreatic cancer?
MRTX1133 targets KRAS G12D, which is present in 35-44% of pancreatic cancers, making it potentially relevant to the largest subset of this highly lethal malignancy. Pancreatic cancer has been notoriously resistant to treatment, with a 5-year survival rate under 10%. MRTX1133 represents the first direct KRAS-targeting approach that has shown significant tumor shrinkage in rigorous preclinical pancreatic cancer models. Phase 1/2 clinical trials are currently enrolling patients.
Q6: How do patients get tested for KRAS mutations?
KRAS mutation testing is now standard for advanced NSCLC, colorectal, and pancreatic cancers. Testing can be performed on tumor tissue through next-generation sequencing (NGS) panels or via liquid biopsy using circulating tumor DNA (ctDNA) from blood samples. Comprehensive NGS panels that identify the specific KRAS variant (not just presence/absence of mutation) are recommended to guide therapy selection.
Q7: What are the main side effects of KRAS inhibitors?
The most common adverse reactions to KRAS G12C inhibitors include diarrhea, musculoskeletal pain, nausea, fatigue, and hepatotoxicity. Laboratory abnormalities include decreased lymphocytes, decreased hemoglobin, and elevated liver enzymes. Most side effects are manageable with dose modifications and supportive care. Combination therapies may have additive toxicities requiring careful monitoring.
Q8: What cancers besides lung, colon, and pancreatic might benefit from KRAS inhibitors?
KRAS mutations occur across multiple cancer types. While less common, they are found in endometrial cancer, biliary tract cancers, gastric cancer, and others. Clinical trials of KRAS inhibitors have included cohorts of patients with various solid tumors harboring specific KRAS mutations. Early data from the KRYSTAL-1 trial showed responses in biliary tract cancers with G12C mutations.
Q9: What is the timeline for broader availability of G12D inhibitors?
MRTX1133 and other G12D-targeted agents are currently in Phase 1/2 clinical trials. Assuming successful completion of these early trials and advancement to Phase 3 studies, FDA approval could potentially occur in 2027-2029. However, patients may access these drugs earlier through clinical trial enrollment or expanded access programs.
Q10: How will KRAS inhibitors impact treatment costs?
While specific pricing hasn't been disclosed for all KRAS inhibitors, approved targeted therapies typically cost $150,000-200,000 annually. However, these drugs may offer value through improved outcomes and quality of life. Combination regimens will increase overall treatment costs but may provide superior efficacy. Access programs and biosimilar competition in the future may affect long-term pricing dynamics.
References
American Cancer Society. (2024). Cancer statistics, 2024. CA: A Cancer Journal for Clinicians, 74(1), 12-49.
Brannon, A. R., Vakiani, E., & Schultz, N. (2025). Clinicogenomic landscape of pancreatic adenocarcinoma identifies KRAS mutant dosage as prognostic of overall survival. Nature Medicine, 31(2), 466-477.
Ebia, M. I., Blais, E. M., Cui, Y., Petricoin, E. F., Pishvaian, M., Gaddam, S., Gong, J., Osipov, A., & Hendifar, A. E. (2025). Evaluating the effect of KRAS variants on survival outcomes and therapy response in pancreatic cancer. JCO Precision Oncology, 9, e2400684.
Food and Drug Administration. (2021, May 28). FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC.
Food and Drug Administration. (2022, December 12). FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC.
Food and Drug Administration. (2024, June 21). FDA grants accelerated approval to adagrasib with cetuximab for KRAS G12C-mutated colorectal cancer.
Food and Drug Administration. (2025, January 16). FDA approves sotorasib with panitumumab for KRAS G12C-mutated colorectal cancer.
Frederick National Laboratory for Cancer Research. (2025, November 19). Unpacking the diversity among KRAS mutants.
Krupa, K., Fudalej, M., Włoszek, E., Miski, H., Badowska-Kozakiewicz, A. M., Mękal, D., Budzik, M. P., Czerw, A., & Deptała, A. (2025). Treatment of KRAS-mutated pancreatic cancer: New hope for the patients? Cancers, 17(15), 2453.
Luo, J. (2023, December 5). MRTX1133 targets tumors with KRAS G12D mutations. National Cancer Institute Cancer Currents Blog.
National Cancer Institute. (2015). Genes co-mutated with KRAS. RAS Dialogue Blog.
National Cancer Institute. (2016). Immunotherapy targets common cancer mutation.
National Cancer Institute. (2020). The disease burden of RAS. RAS Dialogue Blog.
National Cancer Institute. (2021). Sotorasib is first KRAS inhibitor approved by FDA. Cancer Currents Blog.
National Cancer Institute. (n.d.). Mutant KRAS cell lines. RAS Initiative. R
National Library of Medicine. (2023). Study of MRTX1133 in patients with advanced solid tumors harboring a KRAS G12D mutation [Clinical trial registration NCT05737706]. ClinicalTrials.gov.
Prior, I. A., Hood, F. E., & Hartley, J. L. (2020). The frequency of RAS mutations in cancer. Cancer Research, 80(14), 2969-2974.
Stephen, A. G., Esposito, D., Bagni, R. K., & McCormick, F. (2014). Dragging RAS back in the ring. Cancer Cell, 25(3), 272-281. Referenced in National Cancer Institute materials.
U.S. Food and Drug Administration. (2021). Center for Drug Evaluation and Research Application Number: 214665Orig1s000 [Multidiscipline review].
U.S. Food and Drug Administration. (2023). Center for Drug Evaluation and Research Application Number: 216340Orig1s000 [Multidiscipline review].

