The Rise of Multi-Cancer Early Detection (MCED)


Multi-cancer early detection (MCED) technology stands at a pivotal juncture in 2025, with groundbreaking clinical trial data reshaping cancer screening paradigms globally. As the United States projects over 2 million new cancer diagnoses and 618,120 deaths this year, the promise of detecting multiple cancers through a single blood test has captured the attention of regulators, payers, and healthcare systems worldwide.
The Cancer Burden and the Screening Gap
Current Landscape
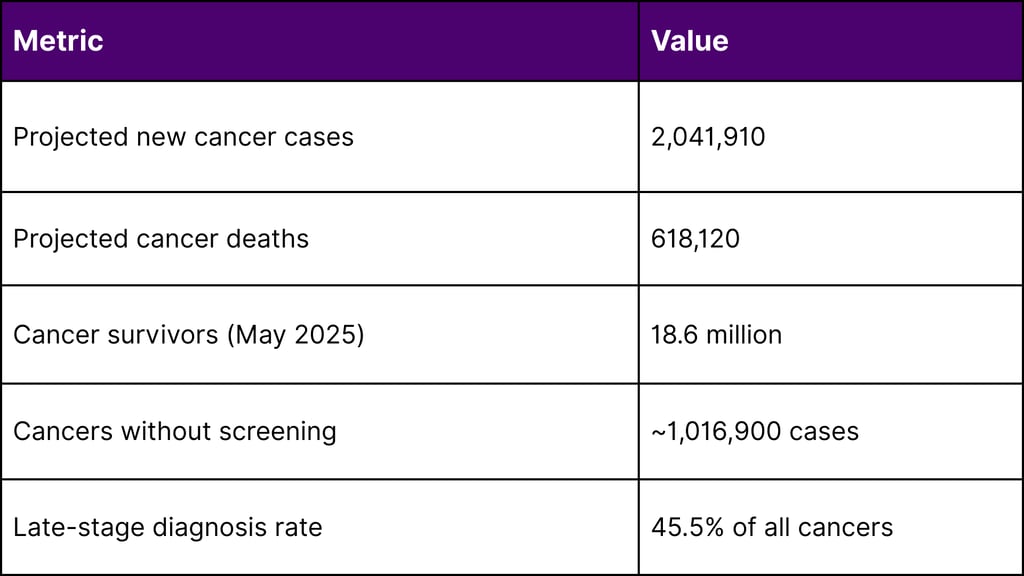
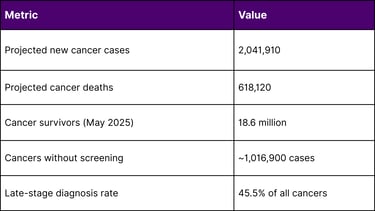
According to the National Cancer Institute, in 2025, an estimated 2,041,910 new cancer cases will be diagnosed in the United States. The cancer mortality rate has continued its decline through 2022, averting nearly 4.5 million deaths since 1991 due to smoking reductions, earlier detection, and improved treatment.
However, a critical gap persists: nearly half of projected cancer diagnoses (approximately 1,016,900 cases) and roughly three-fifths of cancer deaths (358,230) occur in cancers lacking recommended screening protocols. These include pancreatic (13.7% diagnosed at localized stage), ovarian (17.5%), esophageal (21.6%), and liver (40.8%) cancers.
The Promise of MCED Technology
MCED tests analyze biological samples primarily blood for cancer signals using advanced techniques including:
Cell-free DNA and RNA detection
DNA methylation pattern analysis
Protein biomarker identification
Machine learning algorithms for cancer signal origin (CSO) prediction
These tests aim to detect multiple cancer types simultaneously, often before symptoms appear, potentially transforming early detection paradigms.
2025 Landmark Trial Results
PATHFINDER 2 Study: Seven-Fold Cancer Detection Increase
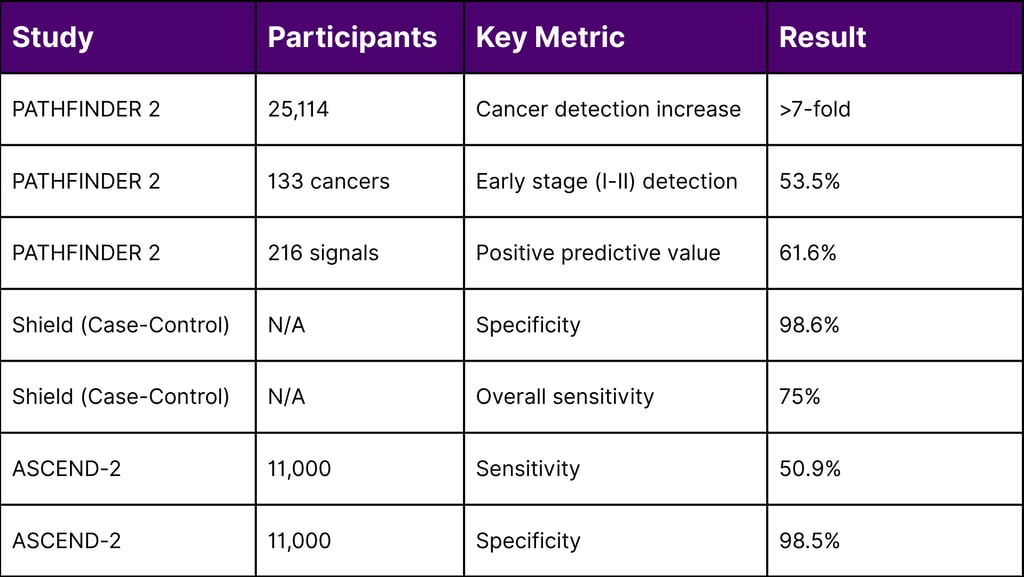
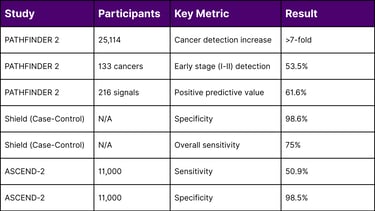
In October 2025, GRAIL announced results from PATHFINDER 2, the largest U.S. MCED interventional study, evaluating the Galleri test in 35,878 participants aged 50 and older.
Key Findings from 25,114 Analyzable Participants:
Cancer Detection Rate: Adding Galleri to standard screening increased cancer detection more than seven-fold
Early-Stage Detection: Over half (53.5%) of Galleri-detected cancers were Stage I or II
Specificity: Maintained high specificity in ruling out cancer
Cancer Signal Origin Accuracy: Successfully predicted cancer location in the majority of positive cases
Safety Profile: No serious study-related adverse events during diagnostic workup
Clinical Significance:
Approximately three-quarters of Galleri-detected cancers lack current recommended screening tests, highlighting the technology's potential to address the screening gap for deadly cancers like pancreatic, esophageal, and ovarian malignancies.
NHS-Galleri Trial: Awaiting 2026 Results
The NHS-Galleri trial represents the world's largest MCED study, enrolling over 140,000 participants aged 50-77 in England. Participants completed three rounds of annual screening through summer 2024.
Trial Design:
Randomized controlled trial (1:1 intervention vs. control)
Primary endpoint: Reduction in late-stage (Stage III-IV) cancer incidence
Secondary analyses: Cancer-specific mortality at five years
Outcomes data collection through summer 2025
Final results expected in 2026
Preliminary Considerations:
NHS England reviewed first-year data in 2024 and determined they were not compelling enough to justify immediate large-scale implementation. The health system will await final 2026 results before considering national rollout, recognizing that first-year trial data often differs from final outcomes.
Additional Clinical Evidence
CancerSEEK (Cancerguard) Program:
Exact Sciences' DETECT-A and ASCEND-2 studies presented updated 2024 data:
ASCEND-2 (11,000 participants): 50.9% sensitivity, 98.5% specificity
Detection focus: Cancers without established screening methods
FDA Breakthrough Device Designation received
Shield Test (Guardant Health):
Presented at 2025 ASCO Annual Meeting:
98.6% specificity across eight cancer types
75% overall cancer sensitivity
Per-cancer sensitivity range: 62-96%
Strong cancer signal origin accuracy
FDA Breakthrough Device Designation granted
Selected for NCI's Vanguard Study
Regulatory Pathways and FDA Positioning
Current Regulatory Status
Critical Regulatory Facts:
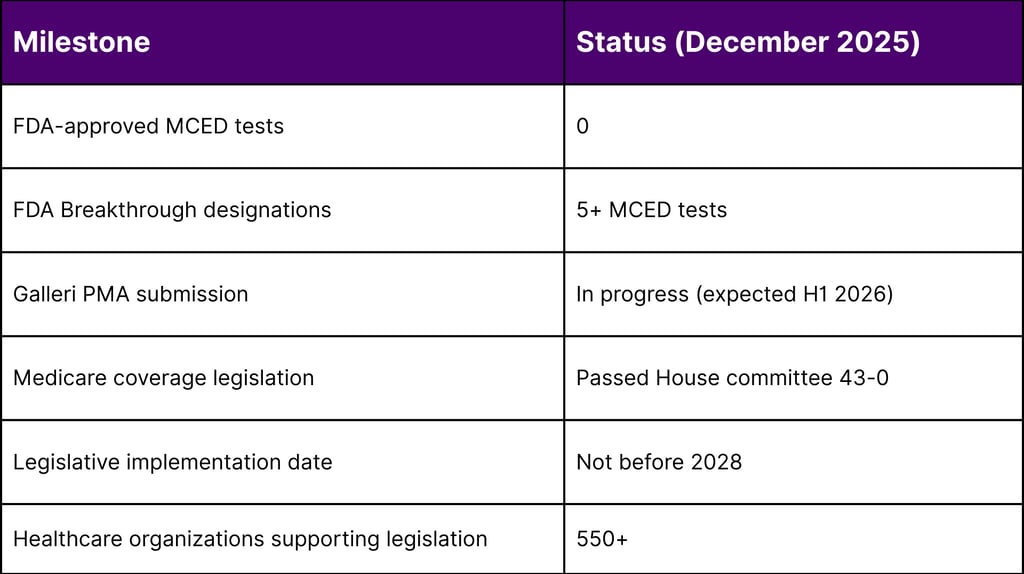
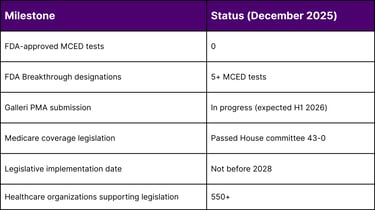
FDA Approval Status: As of December 2025, NO MCED tests have received FDA approval or authorization
Market Access: Tests available as Laboratory Developed Tests (LDTs) under CLIA regulations
Regulatory Requirement: LDTs must demonstrate accurate measurement but are not required to prove clinical benefit
FDA Breakthrough Device Designations
The FDA has granted Breakthrough Device Designation to multiple MCED tests, including:
Galleri (GRAIL): Modular PMA submission in progress, expected completion first half 2026
CancerSEEK/Cancerguard (Exact Sciences): Breakthrough designation received
Shield (Guardant Health): Breakthrough designation granted June 2025
CanScan (Geneseeq): Breakthrough designation for MCED platform
OverC (Burning Rock): Breakthrough designation received 2023
Breakthrough Device Statistics:
As of June 30, 2025, the FDA's Center for Devices and Radiological Health has granted 1,176 Breakthrough Device designations since program inception, with 160 receiving marketing authorization. In fiscal year 2025 (through Q3), 136 breakthrough designations were granted.
Anticipated FDA Timeline
GRAIL's Galleri represents the most advanced regulatory submission, with:
Data from PATHFINDER 2 and NHS-Galleri prevalent screening round
Bridging analyses comparing study versions to commercial version
Expected PMA completion: First half 2026
Potential FDA decision: Late 2026 or 2027
Medicare Coverage and Legislative Momentum
Current Medicare Coverage Landscape
Medicare Part B currently covers preventive screening for limited cancer types:
Breast cancer (mammography)
Cervical and vaginal cancer (Pap tests, pelvic exams)
Colorectal cancer (multiple modalities, including new CT colonography coverage effective January 1, 2025)
Lung cancer (low-dose CT for high-risk individuals)
Prostate cancer (PSA testing)
Critical Gap: No coverage pathway exists for MCED tests, even post-FDA approval.
Medicare MCED Screening Coverage Act
Legislative Status (as of December 2025):
House Bill (H.R. 842): Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act
Introduced: January 31, 2025
House Ways and Means Committee approval: 43-0 vote (September 2025)
Cosponsors: Over 320 House members in previous Congress
Senate Bill (S. 339): Medicare Multi-Cancer Early Detection Screening Coverage Act
Introduced: January 30, 2025
Bipartisan sponsorship: Senate Finance Committee Chair Crapo (R-ID), Ranking Member Wyden (D-OR), members Bennet (D-CO) and Scott (R-SC)
Previous Congress support: 65+ Senate members
Key Legislative Provisions:
Coverage Pathway: Establishes Medicare benefit category for FDA-approved MCED tests
CMS Authority: Authorizes evidence-based coverage determination
Implementation Timeline: Coverage cannot begin before 2028
Payment Structure: Aligned with Cologuard pricing initially, with adjustments after 2031
Existing Screening Protection: Maintains current cancer screening coverage and cost-sharing
Stakeholder Support: Over 550 healthcare organizations endorsing legislation
Budget Considerations:
2028 implementation aligns with anticipated FDA approval timeline
Phased rollout by age group to manage costs
Payment rate structure designed for fiscal responsibility
Market Dynamics and Competitive Positioning
Leading MCED Platforms (December 2025)
1. Galleri (GRAIL)
Technology: Methylation-based cfDNA analysis
Cancer Types: 50+ cancers
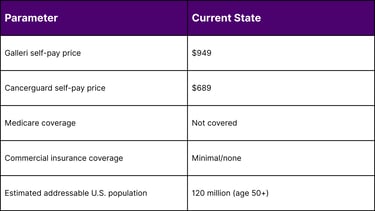
Current Price: $949 (self-pay)
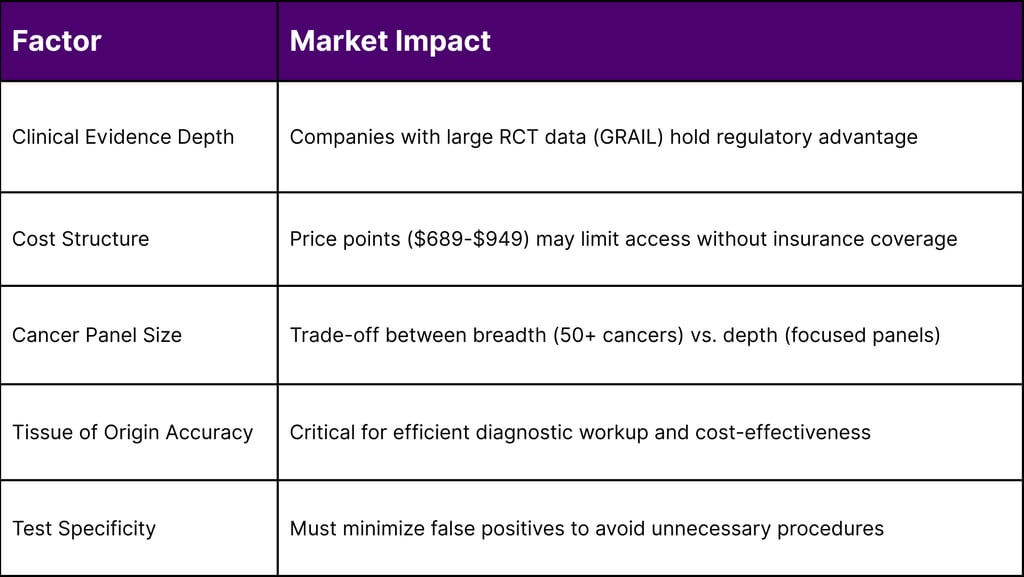
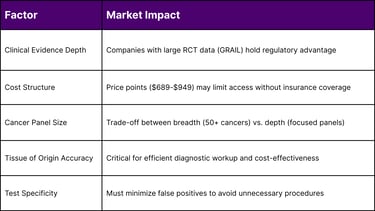
Clinical Evidence: Most extensive (PATHFINDER 2, NHS-Galleri)
Regulatory Status: Breakthrough Device; PMA submission underway
Market Position: First-to-market advantage, strongest clinical data package
2. Cancerguard (Exact Sciences)
Technology: Multi-biomarker approach (DNA mutations, proteins)
Cancer Types: Multiple solid tumors
Price: $689 (self-pay)
Clinical Evidence: DETECT-A, ASCEND-2 studies
Regulatory Status: Breakthrough Device Designation
Market Position: Leverages Exact Sciences' existing screening infrastructure (Cologuard)
3. Shield (Guardant Health)
Technology: Methylation-based
Cancer Types: Eight cancers (bladder, colorectal, esophageal, gastric, liver, lung, ovarian, pancreatic)
Age Group: 45+ years
Clinical Evidence: Case-control validation, NCI Vanguard Study selection
Regulatory Status: Breakthrough Device Designation (June 2025)
Market Position: Strong oncology market presence
4. Asian Innovators
Geneseeq (CanScan): FDA Breakthrough designation, published in Nature Medicine (May 2025)
Burning Rock (OverC): FDA Breakthrough designation for five cancer types
Competitive Differentiators
Payer Sentiment and Reimbursement Outlook
Current Insurance Coverage
Reality Check: As of December 2025:
NO routine insurance coverage for MCED tests
Medicare does not cover MCED tests
Most commercial insurers do not cover MCED screening
Patients pay out-of-pocket ($689-$949 per test)
Payer Evaluation Criteria
Insurers evaluate MCED tests on:
Clinical Utility: Does the test change outcomes (reduced mortality)?
Cost-Effectiveness: Does early detection offset treatment costs?
Evidence Quality: Randomized controlled trial data preferred
False Positive Rate: Impact on downstream diagnostic costs
Integration: Compatibility with existing screening programs
Evidence Requirements
What Payers Want:
Mortality reduction data from RCTs
Cost-effectiveness modeling
Real-world performance data
Demonstrated stage shift (earlier diagnosis)
Long-term outcome tracking
Current Evidence Gaps:
No completed RCTs showing mortality reduction
Limited real-world implementation data
Uncertainty about optimal screening intervals
Questions about population-level impact
Future Reimbursement Scenarios
Scenario 1: FDA Approval + Medicare Coverage (2028+)
Legislative pathway established
Conditional coverage with registry enrollment likely
Phased implementation by age/risk
Estimated 10-20% of eligible population initially
Scenario 2: Selective Commercial Coverage
High-risk populations (genetic predisposition, previous cancer)
Employer self-insured plans
Supplementary to standard screening
Limited market penetration (5-10%)
Scenario 3: Direct-to-Consumer Model
Continued self-pay market
Declining prices through competition
Health savings account (HSA) eligible
Market size constrained by price sensitivity
Global Market Opportunities
United States Market Projection
Addressable Population (2025):
Adults aged 50+: ~120 million
Medicare beneficiaries 65+: ~65 million
Annual screening assumption: 10-30% adoption
Potential annual test volume: 12-36 million tests
Market Value Estimates:
Current self-pay market: <$100 million annually
Post-Medicare coverage (2028+): $5-10 billion potential
5-year market trajectory: Gradual ramp to $15-20 billion (2030)
United Kingdom
NHS Considerations:
Final NHS-Galleri results (2026) critical for rollout decision
UK National Screening Committee evaluation
Potential phased implementation (1 million participants)
Integration with existing NHS screening programs
Cost-per-QALY analysis required
Asia-Pacific Region
Market Drivers:
Rising cancer incidence in aging populations
Government healthcare modernization initiatives
Domestic MCED developers (China, Japan, South Korea)
Growing middle class with healthcare purchasing power
Regional Challenges:
Reimbursement infrastructure varies widely
Regulatory approval timelines differ by country
Competition from local developers
Price sensitivity in many markets
European Union
Market Characteristics:
Country-specific healthcare systems
Stringent evidence requirements
CE marking pathway for commercialization
Integration with national screening programs
Cost-effectiveness thresholds vary by country
Clinical Integration and Implementation Challenges
Healthcare System Considerations
Diagnostic Workup Infrastructure:
Positive MCED results require diagnostic evaluation
CT/PET-CT scanning capacity
Specialty referral pathways
Potential strain on imaging and endoscopy services
Primary Care Physician Readiness:
Education on test interpretation
Counseling patients on results
Managing false positive anxiety
Coordinating diagnostic workup
Health Equity Concerns:
Access disparities without insurance coverage
Geographic availability (rural vs. urban)
Socioeconomic barriers to follow-up care
Racial and ethnic representation in trials
Psychological and Ethical Considerations
Patient Impact:
Anxiety from false positive results
Cancer signal detected but no cancer found scenarios
Decision-making complexity
Screening fatigue
Ethical Questions:
Appropriate age for screening initiation
Overdiagnosis potential
Resource allocation priorities
Informed consent processes
Technical and Analytical Advancements
Technology Evolution (2025)
Next-Generation Improvements:
Enhanced sensitivity for early-stage cancers
Improved tissue of origin prediction algorithms
Reduced blood sample volume requirements
Faster turnaround times (results in 10-14 days)
Artificial Intelligence Integration:
Machine learning for pattern recognition
Integration of multi-omics data
Predictive modeling for risk stratification
Continuous algorithm refinement
Ongoing Research Directions
NCI Cancer Screening Research Network (CSRN):
Vanguard study launched 2025 (24,000 participants)
Evaluating multiple MCED technologies
Feasibility assessment for larger trials
Expected to inform future RCT design
Industry-Sponsored Studies:
Long-term follow-up of trial participants
Real-world evidence collection
Health economics research
Comparative effectiveness studies
Key Statistics and Market Data
Cancer Burden (2025 U.S. Data - NCI/CDC)
MCED Clinical Performance (2025 Trial Data)
Regulatory and Legislative Status
Market Pricing and Access
Expert Perspectives
Clinical Community Views
Physicians and oncologists express cautious optimism about MCED technology. The potential to detect cancers lacking screening programs pancreatic, ovarian, esophageal generates significant interest. However, concerns about false positives, patient anxiety, and resource strain on diagnostic services temper enthusiasm.
Primary care physicians require clear guidance on test interpretation, patient counseling, and workup protocols. Professional medical societies await final trial data before issuing screening recommendations.
Patient Advocacy Position
Cancer advocacy organizations strongly support MCED technology development and Medicare coverage legislation. Over 550 organizations have endorsed the Medicare Multi-Cancer Early Detection Screening Coverage Act, recognizing the potential to save lives through earlier detection.
Patient advocates emphasize the importance of equitable access, noting that out-of-pocket costs ($689-$949) create barriers for many Americans. Medicare coverage represents a critical step toward democratizing access to this technology.
Regulatory Perspective
The FDA's Breakthrough Device Designation for multiple MCED tests signals recognition of their potential to address unmet medical needs. However, the agency maintains rigorous standards for safety and effectiveness evidence.
The modular PMA submission approach allows manufacturers to submit data components progressively, potentially accelerating review timelines while maintaining regulatory thoroughness.
Payer Considerations
Insurance payers remain in evidence-gathering mode, waiting for definitive clinical utility data. While early trial results show promise, payers require mortality reduction evidence from completed randomized controlled trials.
Cost-effectiveness analyses will play a crucial role in coverage decisions. Payers must balance the potential benefits of earlier cancer detection against the costs of the tests themselves and downstream diagnostic procedures.
Future Outlook: 2026-2030
Near-Term Milestones (2026-2027)
2026:
NHS-Galleri final results publication (expected mid-2026)
Galleri FDA PMA submission completion (H1 2026)
NCI CSRN Vanguard study preliminary data
Additional commercial MCED test launches
2027:
Potential FDA approval of first MCED test
Medicare legislation passage potential
Commercial insurance coverage decisions
International regulatory approvals (UK, EU)
Medium-Term Evolution (2028-2030)
2028:
Medicare coverage implementation (earliest per legislation)
Large-scale real-world evidence generation
Health economics data accumulation
Competitive market dynamics intensification
2029-2030:
Multiple FDA-approved MCED tests in market
Commercial insurance coverage expansion
Price competition driving costs down
Integration with electronic health records
Population health management programs
Long-Term Transformation
Potential Paradigm Shifts:
Personalized Screening Protocols: Risk-stratified approaches using MCED in combination with traditional screening
Annual Multi-Cancer Checkup: MCED tests becoming routine part of preventive care (age 50+)
Healthcare Cost Reallocation: Shift from late-stage treatment to early detection investment
Cancer Mortality Reduction: Potential 10-20% reduction in cancer deaths by 2035-2040
Global Health Impact: MCED technology adaptation for resource-limited settings
Strategic Considerations for Stakeholders
For Healthcare Systems
Action Items:
Monitor trial results and regulatory developments
Plan diagnostic capacity expansion
Develop MCED result management protocols
Train primary care workforce
Establish cancer navigation pathways
For Payers
Evaluation Framework:
Establish clinical evidence review committees
Conduct cost-effectiveness modeling
Design coverage-with-evidence-development programs
Develop prior authorization criteria
Plan phased rollout strategies
For MCED Developers
Competitive Imperatives:
Complete rigorous clinical validation studies
Pursue regulatory approvals aggressively
Build strategic healthcare partnerships
Invest in patient/physician education
Develop value-based contracting models
For Policy Makers
Legislative Priorities:
Advance Medicare coverage legislation
Support cancer research funding (NCI)
Address health equity in screening access
Facilitate data sharing for research
Monitor market competition dynamics
Conclusion
The multi-cancer early detection field stands at an inflection point in late 2025. Landmark clinical trial results from PATHFINDER 2 demonstrate the technology's potential to dramatically increase cancer detection rates, particularly for deadly cancers lacking current screening options. With over seven times more cancers detected when MCED testing supplements standard screening, the clinical promise is undeniable.
However, significant hurdles remain. No MCED test has achieved FDA approval, and without Medicare coverage legislation, access will remain limited to those who can afford out-of-pocket costs. The NHS-Galleri trial results, expected in 2026, will provide crucial evidence on whether MCED screening can achieve its ultimate goal: reducing late-stage cancer diagnoses and cancer mortality.
The next 3-5 years will determine whether MCED technology fulfills its transformative potential or becomes a promising innovation that struggles with real-world implementation. Regulatory approval, reimbursement decisions, healthcare system integration, and long-term outcome data will shape the trajectory of this field.
For the approximately 1 million Americans diagnosed annually with cancers lacking screening tests many at late stages when prognosis is poor MCED technology represents hope. Whether that hope translates into improved survival rates depends on successfully navigating the complex intersection of science, regulation, reimbursement, and healthcare delivery.
The rise of multi-cancer early detection in 2025 marks not an endpoint, but the beginning of a potentially revolutionary chapter in cancer care. The global screening market is watching closely as trial data accumulates, regulatory pathways advance, and legislative momentum builds toward making this technology accessible to those who need it most.
Frequently Asked Questions (FAQ)
Q1: Are MCED tests FDA-approved?
No. As of December 2025, no multi-cancer early detection test has received FDA approval or authorization. Several tests, including Galleri, Cancerguard, and Shield, have received FDA Breakthrough Device Designation, which expedites the review process but does not constitute approval. These tests are currently available as Laboratory Developed Tests (LDTs) under CLIA regulations.
Q2: Does Medicare cover MCED tests?
No. Medicare does not currently cover MCED tests. However, the Medicare Multi-Cancer Early Detection Screening Coverage Act (H.R. 842/S. 339) passed the House Ways and Means Committee in September 2025 with bipartisan 43-0 support. If enacted, the legislation would establish a Medicare coverage pathway for FDA-approved MCED tests, with implementation not before 2028.
Q3: How much do MCED tests cost?
Current self-pay prices range from $689 (Cancerguard by Exact Sciences) to $949 (Galleri by GRAIL). Without insurance coverage, patients must pay these costs out-of-pocket. Health savings accounts (HSAs) and flexible spending accounts (FSAs) may be used for payment in some cases.
Q4: How accurate are MCED tests?
Accuracy varies by test and cancer type. From 2025 trial data:
Galleri (PATHFINDER 2): 61.6% positive predictive value (meaning 61.6% of positive results led to cancer diagnosis), with 53.5% of detected cancers at early stages (I-II)
Shield (case-control): 98.6% specificity (accurate in ruling out cancer) and 75% overall sensitivity across eight cancer types
Cancerguard (ASCEND-2): 50.9% sensitivity and 98.5% specificity
Importantly, these tests do not detect all cancers, and false positives occur, requiring follow-up diagnostic procedures.
Q5: What happens if my MCED test is positive?
A positive MCED test indicates a cancer signal was detected. This does NOT mean you definitively have cancer. Follow-up diagnostic testing is required, which may include:
Additional blood tests
CT or PET-CT scans
Organ-specific imaging
Endoscopy or colonoscopy
Biopsy procedures
The MCED test typically predicts a "cancer signal origin" (CSO) to guide diagnostic workup toward specific organs or tissues.
Q6: Should I get an MCED test?
This decision should be made in consultation with your healthcare provider. Considerations include:
Your age (most trials focused on 50+ years)
Personal and family cancer history
Adherence to standard cancer screening recommendations
Financial ability to pay out-of-pocket
Emotional readiness to handle uncertain results
Availability of follow-up diagnostic services
The U.S. Preventive Services Task Force has not issued recommendations on MCED tests, and professional medical societies await more evidence before providing guidance.
Q7: Do MCED tests replace standard cancer screenings?
No. MCED tests are designed to supplement, not replace, existing cancer screenings like mammograms, colonoscopy, and low-dose CT for lung cancer. All individuals should continue to follow guideline-recommended screening for breast, cervical, colorectal, and lung cancers (if eligible).
Q8: When will the NHS-Galleri trial results be available?
The NHS-Galleri trial, which enrolled over 140,000 participants in England, completed final study visits in summer 2024. Outcomes data collection continues through summer 2025, with final results expected to be published in 2026. These results will inform whether the UK National Health Service implements MCED screening at a national scale.
Q9: Can MCED tests detect all types of cancer?
No. Different MCED tests detect different numbers of cancer types:
Galleri: Claims to detect signals from 50+ cancer types
Shield: Focuses on eight cancers (bladder, colorectal, esophageal, gastric, liver, lung, ovarian, pancreatic)
Cancerguard: Targets multiple solid tumors
Even the broadest MCED tests do not detect all cancer types, and sensitivity varies significantly by cancer type and stage.
Q10: What is the next major milestone for MCED tests?
The most immediate significant milestone is GRAIL's expected completion of its Premarket Approval (PMA) submission to the FDA in the first half of 2026. This will include data from the PATHFINDER 2 study and the prevalent screening round of the NHS-Galleri trial. FDA review and potential approval would likely occur in late 2026 or 2027, representing a critical step toward broader clinical adoption and insurance coverage.
References
National Cancer Institute. (2025). Cancer Statistics.
Centers for Disease Control and Prevention. (2025). U.S. Cancer Statistics Public Use Database.
Siegel, R. L., Kratzer, T. B., Giaquinto, A. N., Sung, H., & Jemal, A. (2025). Cancer statistics, 2025. CA: A Cancer Journal for Clinicians, 75(1), 10-45. doi:10.3322/caac.21871
National Cancer Institute. (2025). Annual Report to the Nation on the Status of Cancer.
National Institutes of Health. (2025, April 21). Annual Report to the Nation: Cancer deaths continue to decline.
National Cancer Institute, Division of Cancer Prevention. (2025). Questions and Answers about Multi-Cancer Detection Tests.
U.S. Food and Drug Administration. (2025). Breakthrough Devices Program.
Centers for Medicare & Medicaid Services. (2025). Preventive Services Coverage.
Medicare.gov. (2025). Your Guide to Medicare Preventive Services.
U.S. Senate. (2025). S. 339 - Medicare Multi-Cancer Early Detection Screening Coverage Act. 119th Congress.
U.S. House of Representatives. (2025). H.R. 842 - Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act. 119th Congress.
U.S. Senator Michael Bennet. (2025, February 6). Bennet, Colleagues Reintroduce Legislation to Ensure Medicare Beneficiaries Receive Coverage for Cancer Detection Technologies.
U.S. Senator Mike Crapo. (2025, February 3). Finance Committee Members Introduce Bipartisan Legislation to Ensure Medicare Patients' Access to Cancer Detection Technologies.
Division of Cancer Control and Population Sciences, National Cancer Institute. (2025). Statistics and Graphs.
Hoffman, R. M., Wolf, A. M. D., Raoof, S., et al. (2025). Multicancer early detection testing: Guidance for primary care discussions with patients. Cancer, 131, e35823. doi:10.1002/cncr.35823
Imai, M., Nakamura, Y., & Yoshino, T. (2025). Transforming cancer screening: The potential of multi-cancer early detection (MCED) technologies. International Journal of Clinical Oncology, 30(2), 180-193. doi:10.1007/s10147-025-02694-5
Swanton, C., Bachtiar, V., Mathews, C., et al. (2025). NHS-Galleri trial: Enriched enrolment approaches and sociodemographic characteristics of enrolled participants. Clinical Trials, 22(2), 227-238. doi:10.1177/17407745241302477
Lowenhoff, I., Dolly, S., Dowinton Smith, R., et al. (2025). Clinical referral to the NHS following multi-cancer early detection test results from the NHS-Galleri trial. Frontiers in Oncology, 15. doi:10.3389/fonc.2025.1511816
American Cancer Society. (2025). Multi-cancer Detection (MCD) Tests.
American Cancer Society Cancer Action Network. (2025). Legislation Reintroduced in the House and Senate Aimed at Increasing Early Cancer Detection in Medicare.
Prevent Cancer Foundation. (2025). Multi-Cancer Early Detection Coverage & Legislation.
GRAIL, Inc. (2025, October 17). GRAIL PATHFINDER 2 Results Show Galleri® Multi-Cancer Early Detection Blood Test Increased Cancer Detection More Than Seven-Fold. Press Release.
Exact Sciences Corporation. (2025). Cancerguard Multi-Cancer Early Detection Test.
Guardant Health. (2025, June 2). Guardant Health Shield™ Test Receives FDA Breakthrough Device Designation for Blood-Based Multi-Cancer Early Detection. Press Release.
Alliance for the Advancement of Multi-Cancer Early Detection. (2025). Multi-Cancer Early Detection Technology Overview.

