The TIL Revolution


A New Era in Oncology Immunotherapy
After more than three decades of dedicated research, tumor-infiltrating lymphocyte (TIL) therapy has emerged from the laboratory to become a game-changing reality in solid tumor treatment. In February 2024, the U.S. Food and Drug Administration granted accelerated approval to lifileucel (Amtagvi), marking a historic milestone as the first TIL therapy and the first cellular therapy of any kind approved for treating a solid tumor.
This breakthrough represents far more than a single drug approval. It signals the dawn of a new paradigm in oncology, where personalized immune cell therapies harness the body's natural cancer-fighting capabilities to achieve durable responses in patients who have exhausted conventional treatment options.
Understanding TIL Therapy: Harnessing the Body's Cancer Fighters
The Science Behind TILs
Tumor-infiltrating lymphocytes are immune cells primarily T cells that naturally migrate into tumors in an attempt to mount an immune response against cancer. These specialized cells have already demonstrated their ability to recognize tumor-specific antigens and navigate the challenging tumor microenvironment. However, in most cancer patients, TILs exist in insufficient numbers and often become exhausted by the immunosuppressive conditions within tumors.
TIL therapy addresses these limitations through a sophisticated manufacturing process:
Tumor Harvest: Surgeons collect a tumor sample from the patient through biopsy or surgery
TIL Isolation: Laboratory technicians isolate lymphocytes from the tumor tissue
Expansion: The isolated TILs are cultured with interleukin-2 (IL-2) to expand their numbers from thousands to billions
Patient Preparation: Patients receive lymphodepleting chemotherapy to create an optimal environment for the infused TILs
Infusion: The expanded TILs are infused back into the patient as a one-time treatment
Support: Post-infusion IL-2 administration further enhances TIL activity and expansion in vivo
What Makes TIL Therapy Unique
Unlike CAR T-cell therapy, which requires genetic engineering of T cells collected from blood, TIL therapy utilizes cells already present within the tumor without genetic modification. These naturally occurring tumor-infiltrating cells possess inherent advantages:
Tumor Recognition: TILs have already proven their ability to identify and target tumor cells
Tumor Penetration: These cells have successfully navigated into solid tumor tissue
Polyclonal Response: TILs recognize multiple tumor antigens, potentially providing more comprehensive anti-tumor activity
Neoantigen Targeting: Research has demonstrated that effective TILs primarily target patient-specific neoantigens unique proteins created by tumor mutations rather than normal tissue antigens
The Historic FDA Approval: Lifileucel for Advanced Melanoma
Clinical Evidence Supporting Approval
The FDA's accelerated approval of lifileucel was based on compelling data from the C-144-01 clinical trial. Among 73 patients with advanced melanoma who received the approved dose of at least 7.5 billion cells:
Objective Response Rate: Nearly one-third of patients experienced tumor reduction
Complete Responses: Several patients achieved complete disappearance of all detectable tumors
Durable Responses: Approximately 40% of responding patients maintained disease control for at least one year after a single infusion
Treatment Setting: All patients had previously progressed on PD-1/PD-L1 immune checkpoint inhibitors and, if BRAF-mutated, BRAF inhibitors
These results are particularly remarkable given that patients had exhausted multiple prior therapies, including the current standard-of-care immunotherapies.
The Melanoma Burden in the United States
Melanoma represents a significant public health challenge:
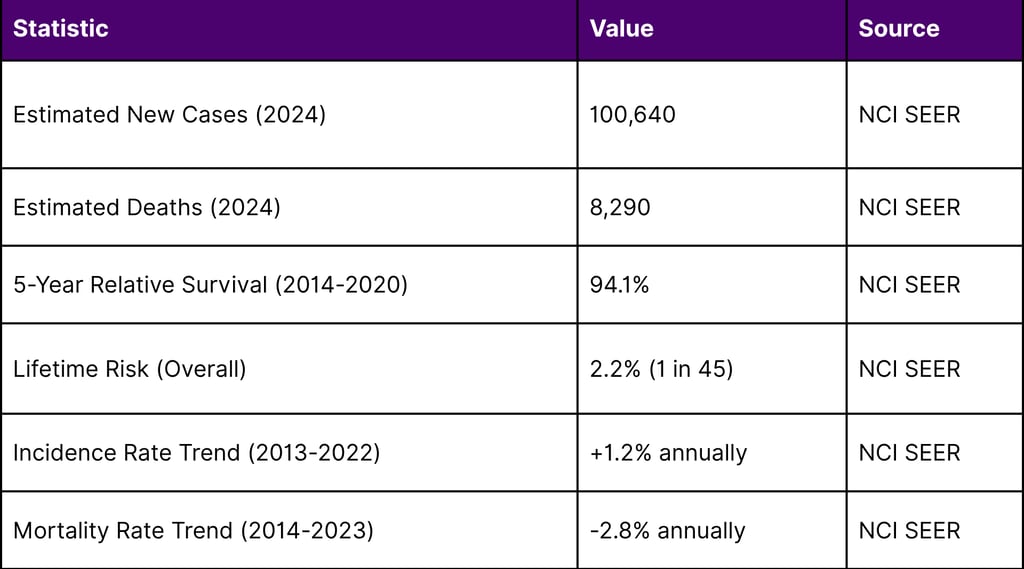
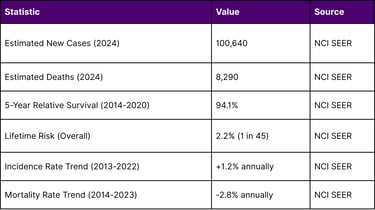
The declining mortality rate despite rising incidence reflects significant advances in melanoma treatment, particularly with immune checkpoint inhibitors and now TIL therapy.
Beyond Melanoma: Expanding TIL Applications
Cervical Cancer Shows Promise
Cervical cancer has emerged as another promising indication for TIL therapy, driven by the viral etiology of most cases. Human papillomavirus (HPV)-associated cervical cancers express viral oncoproteins E6 and E7, providing attractive targets for TIL-based immunotherapy.
Clinical Trial Results in Cervical Cancer
Clinical trials have demonstrated encouraging efficacy signals:
Phase II Study (LN-145): In patients with recurrent cervical cancer after chemotherapy:
Objective Response Rate: 44%
Disease Control Rate: 89%
Complete Response Rate: 11.1%
Early Trials (NCT01585428): Among 18 HPV-positive cervical cancer patients:
Objective Response Rate: 27.8%
Complete Responses: 2 patients achieved ongoing complete remissions lasting over 5 years
The FDA granted Breakthrough Therapy designation to LN-145 for advanced cervical cancer, expediting its development pathway.
Ongoing Clinical Investigations
Multiple clinical trials registered on ClinicalTrials.gov are evaluating TIL therapy across various solid tumors:
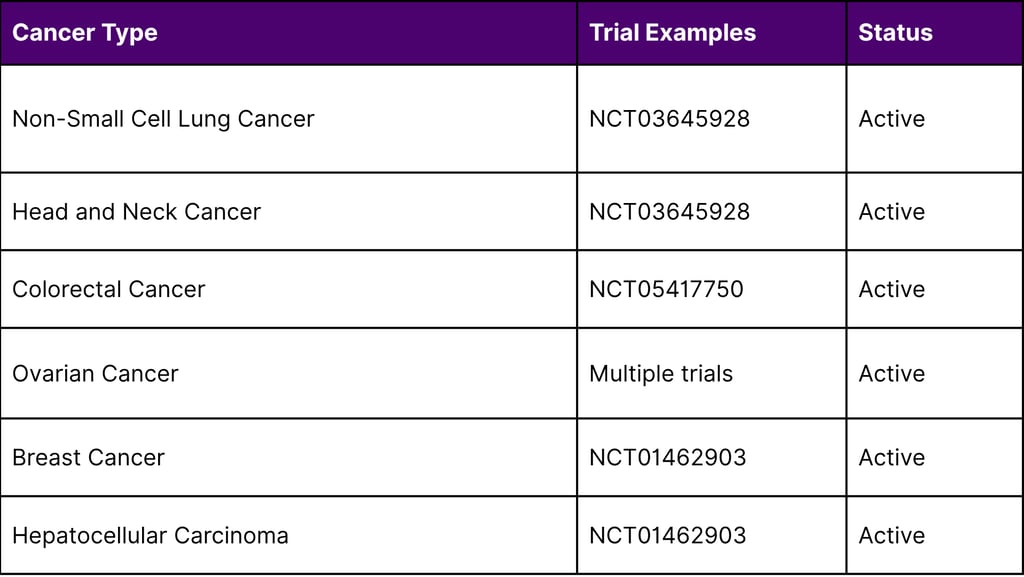
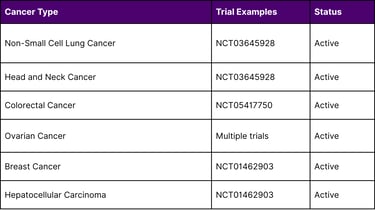
Research at the National Cancer Institute has documented complete tumor regression in individual patients with advanced colon cancer and breast cancer, demonstrating TIL therapy's potential beyond melanoma and cervical cancer.
The 30-Year Journey: From Laboratory Discovery to FDA Approval
Pioneering Research at the National Cancer Institute
The TIL therapy story begins in the 1980s with Steven Rosenberg, M.D., Ph.D., and colleagues at the National Cancer Institute's Surgery Branch. In the late 1980s, Dr. Rosenberg led the first clinical trials demonstrating that TIL therapy could shrink tumors in patients with very advanced melanoma.
Key milestones in TIL development:
1987: First identification of specific cytolytic immune responses against autologous tumors
Late 1980s: First clinical trials of TIL therapy in advanced melanoma
2011: NCI entered cooperative research agreement with Iovance Biotherapeutics to advance TIL therapy development
2015: First reported clinical trial of TIL therapy in cervical cancer
February 16, 2024: FDA approves lifileucel for advanced melanoma
Understanding the Mechanisms
Laboratory studies have elucidated critical mechanisms underlying TIL therapy efficacy:
Lymphodepleting Conditioning Regimen: The preparative chemotherapy before TIL infusion enhances anti-tumor activity through three mechanisms:
Depleting regulatory T cells that suppress immune responses
Eliminating competing lymphocytes, allowing homeostatic cytokines (IL-7, IL-15) to preferentially support infused TILs
Damaging mucosal barriers, leading to increased toll-like receptor ligands that stimulate antigen-presenting cells
Neoantigen Recognition: A breakthrough discovery revealed that effective TILs primarily recognize neoantigens tumor-specific mutations rather than normal tissue proteins. This explains why TIL therapy typically avoids the autoimmune toxicity seen when T cells target normal tissue antigens.
Market Dynamics and R&D Investment
The Growing TIL Therapy Pipeline
Analysis of ClinicalTrials.gov data reveals significant momentum in TIL therapy research:
Total TIL Clinical Trials: Over 200 trials registered as of 2024
Primary Focus: Melanoma represents the largest proportion of trials
Geographic Distribution: Trials conducted across North America, Europe, and Asia
Combination Approaches: Increasing number of trials combining TILs with checkpoint inhibitors
Combination Therapy Strategies
The IOV-COM-202 phase 2 trial evaluated lifileucel combined with pembrolizumab (a PD-1 inhibitor) in patients with advanced melanoma who had not received prior checkpoint inhibitor therapy:
Overall Response Rate: 67%
Implication: Combination therapy may enhance efficacy in first-line settings
The TILVANCE-301 phase 3 trial is currently investigating this combination to potentially establish a new first-line treatment paradigm.
Clinical Implementation Considerations
Manufacturing and Logistics
TIL therapy manufacturing involves sophisticated logistics:
Tumor Collection: Performed at the treating hospital
Manufacturing: Tumor samples sent to centralized facilities for TIL expansion (typically 3-4 weeks)
Quality Control: Potency assessment through interferon-gamma release assays
Minimum Cell Dose: At least 1 billion TILs required (approved dose: ≥7.5 billion)
Cryopreservation: TIL products can be cryopreserved for future infusion
Patient Selection and Management
Optimal TIL therapy candidates:
Patients with measurable solid tumors amenable to surgical harvest
Previous progression on standard therapies
Adequate organ function to tolerate lymphodepleting chemotherapy
Performance status compatible with intensive immunotherapy
Safety Profile
Common adverse events associated with TIL therapy include:
Hematologic toxicity from lymphodepleting chemotherapy
Cytokine-related effects from IL-2 administration (fever, capillary leak syndrome)
Immune-related adverse events (generally less severe than with CAR T-cell therapy)
The toxicity profile is generally manageable, and dose modifications of IL-2 can reduce treatment-related complications while maintaining efficacy.
Future Directions: Next-Generation TIL Therapies
Genetic Modification Strategies
Researchers are developing genetically modified TILs (GM-TILs) to overcome current limitations:
CRISPR-Based Modifications:
Knockout of inhibitory checkpoint receptors (e.g., PD-1, CTLA-4)
Knockout of immunosuppressive signaling pathways
Enhancement of cytokine signaling
Engineered Features:
Insertion of high-affinity T-cell receptors targeting specific tumor antigens
Addition of stem-like markers to improve TIL persistence
Expression of cytokines to enhance anti-tumor activity
Optimizing TIL Selection
Advanced characterization techniques enable identification of the most potent TIL subpopulations:
Neoantigen-Reactive TILs: Selection of cells recognizing patient-specific tumor mutations
Phenotypic Selection: Enrichment of TILs with memory or stem-like properties
Single-Cell Analysis: High-throughput sequencing to identify optimal T-cell receptors
Reducing Manufacturing Time
Current TIL manufacturing requires several weeks. Strategies to accelerate production include:
Rapid Expansion Protocols: Shortened culture periods while maintaining cell quality
Automated Systems: Closed-system bioreactors for standardized manufacturing
Alternative Expansion Methods: Use of antibody agonists (e.g., anti-CD137) to replace feeder cells
Comparative Analysis: TIL vs. Other Cellular Therapies
TIL Therapy vs. CAR T-Cell Therapy
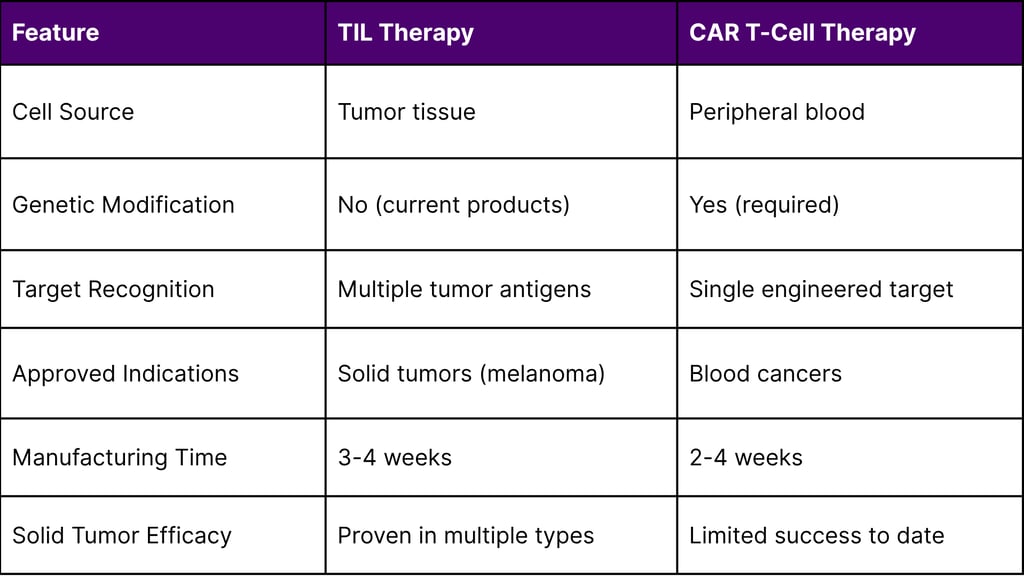
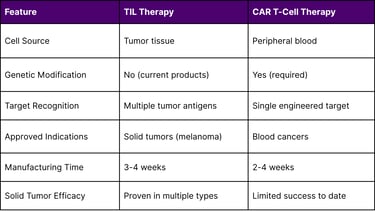
Advantages of TIL Therapy for Solid Tumors
Natural Tumor Infiltration: TILs have already demonstrated ability to penetrate solid tumor barriers
Polyclonal Response: Recognition of multiple antigens provides comprehensive coverage
No Genetic Engineering Required: Reduces manufacturing complexity and regulatory considerations
Neoantigen Targeting: Reduced risk of on-target/off-tumor toxicity affecting normal tissues
Economic and Access Considerations
Pricing and Reimbursement
While specific pricing for lifileucel has not been disclosed publicly by Iovance, cellular therapies typically command premium pricing reflecting:
Complex manufacturing processes
Personalized medicine approach
Intensive patient monitoring requirements
Potential for durable, one-time treatment benefit
Healthcare System Implications
Successful TIL therapy implementation requires:
Specialized Treatment Centers: Facilities capable of performing tumor harvests and managing cellular therapy complications
Manufacturing Infrastructure: Centralized facilities with quality control systems
Multidisciplinary Teams: Surgeons, medical oncologists, immunotherapy specialists, and pharmacists
Patient Support Services: Coordination of logistics, housing for out-of-area patients, and follow-up care
Statistical Insights: TIL Therapy Impact
Response Duration Analysis
Data from melanoma trials demonstrate remarkable durability:
TIL Therapy Response Duration in Advanced Melanoma
- Median Response Duration: Not yet reached in many trials
- 1-Year Progression-Free Survival (Responders): ~40%
- Complete Response Rate: 5-10% across studies
- Complete Response Duration: Many patients maintaining remission >5 years
Comparative Efficacy Metrics
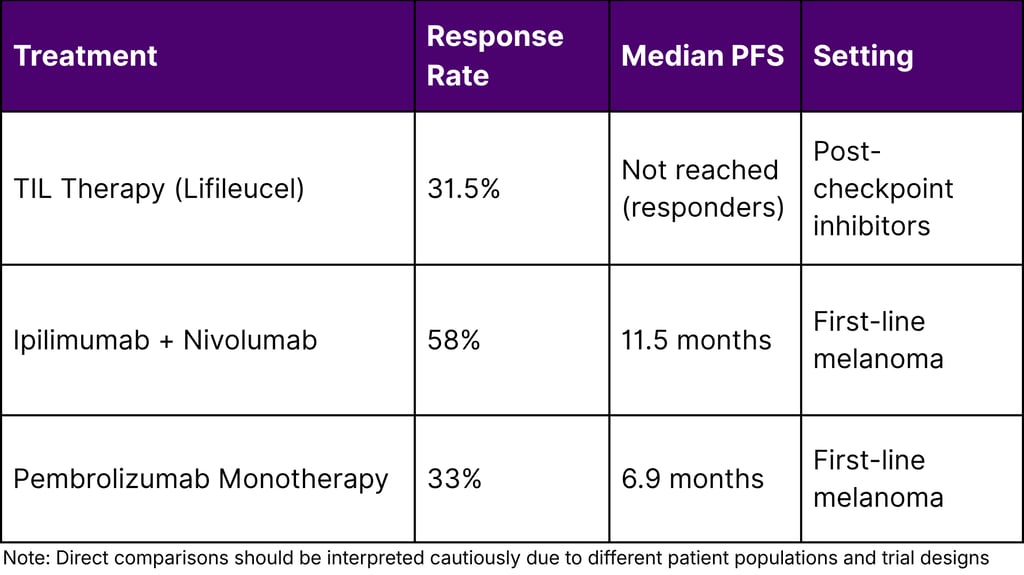
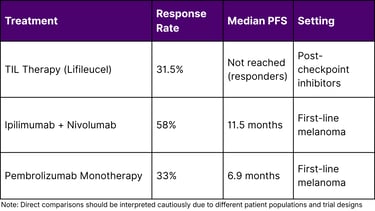
Challenges and Opportunities
Current Limitations
Manufacturing Complexity:
TIL expansion requires 3-4 weeks, limiting treatment of rapidly progressing patients
Not all tumor samples successfully generate sufficient TILs
Manufacturing costs remain substantial
Patient Selection:
Requires accessible tumor for harvest
Patients must have adequate performance status to tolerate treatment
Some tumors contain insufficient TILs or highly suppressive microenvironments
Clinical Implementation:
Limited number of centers with TIL therapy expertise
Reimbursement mechanisms still developing
Lack of predictive biomarkers to identify optimal candidates
Opportunities for Advancement
Biomarker Development:
Identification of predictive markers for TIL therapy response
Characterization of optimal TIL product attributes
Assessment of tumor microenvironment factors influencing efficacy
Combination Strategies:
Integration with checkpoint inhibitors to enhance activity
Combination with targeted therapies or radiation
Sequential treatment approaches optimizing timing and sequencing
Manufacturing Innovation:
Automation to improve consistency and reduce costs
Alternative expansion methods to accelerate production
Development of "off-the-shelf" allogeneic approaches (though challenging for TILs)
Global Perspective: TIL Therapy Development
International Clinical Activity
While the United States leads in TIL therapy development, significant research occurs globally:
Europe: Multiple centers conducting TIL trials, particularly in the Netherlands, Denmark, and the United Kingdom
Asia: Growing interest in China and Japan, with several domestic TIL programs emerging
Australia: Active participation in international trials and domestic research initiatives
Regulatory Pathways
The FDA's accelerated approval pathway enabled lifileucel's authorization based on objective response rate, with continued approval contingent on confirmatory trials. This approach balances timely patient access with rigorous evidence generation.
Other regulatory agencies are evaluating similar frameworks for cellular therapies:
European Medicines Agency (EMA): Conditional marketing authorization pathway
Japan's PMDA: Regenerative medicine products framework
China's NMPA: Priority review for innovative cellular therapies
Patient Perspectives: Living with TIL Therapy
Treatment Journey
Patients undergoing TIL therapy experience a multi-phase process:
Phase 1 - Tumor Harvest (Week 0):
Surgical procedure to obtain tumor tissue
Brief recovery period
Tumor sent for TIL manufacturing
Phase 2 - Manufacturing (Weeks 1-4):
Patient recovers while TILs expand in laboratory
Baseline assessments and treatment planning
Patient education about upcoming phases
Phase 3 - Lymphodepletion (Week 5):
5-7 days of preparative chemotherapy
Hospitalization required
Monitoring for chemotherapy toxicity
Phase 4 - TIL Infusion (Week 5):
Single infusion of billions of TILs
Generally well-tolerated infusion procedure
Initiation of IL-2 support therapy
Phase 5 - Recovery and IL-2 (Weeks 5-6):
Multiple doses of IL-2 to support TIL expansion
Management of IL-2-related side effects
Gradual recovery and hospital discharge
Phase 6 - Follow-Up (Months 1-12+):
Regular imaging to assess tumor response
Long-term monitoring for efficacy and safety
Potential for durable disease control after single treatment
Quality of Life Considerations
TIL therapy offers potential advantages for quality of life:
One-Time Treatment: No ongoing therapy required if durable response achieved
Defined Treatment Period: Acute toxicity phase followed by recovery
Absence of Chronic Toxicity: Unlike continuous therapies, no long-term daily treatment burden
Investment and Business Landscape
Key Players in TIL Therapy
Iovance Biotherapeutics:
Pioneer in TIL commercialization
Lifileucel (Amtagvi) approved for melanoma
Expanding pipeline in multiple solid tumors
Academic Institutions:
National Cancer Institute (continuing groundbreaking research)
MD Anderson Cancer Center
Moffitt Cancer Center
Multiple international academic centers
Emerging Companies:
Several biotechnology companies developing next-generation TIL approaches
Focus on manufacturing optimization, genetic modification, and combination strategies
Market Opportunity
The solid tumor immunotherapy market represents a substantial opportunity:
Global oncology immunotherapy market projected to exceed $200 billion by 2030
TIL therapy positioned to capture share in multiple indications
Potential expansion beyond current approved indication as evidence accumulates
Conclusion: A Transformative Moment in Oncology
The February 2024 FDA approval of lifileucel represents far more than a new treatment option it validates three decades of scientific persistence and marks the beginning of a new era in solid tumor immunotherapy. The TIL revolution demonstrates that cellular therapy can succeed where traditional approaches have struggled: in the treatment of solid tumors.
Several factors position TIL therapy for continued growth and impact:
Proven Efficacy: Clinical data demonstrate meaningful responses in heavily pretreated patients
Expanding Evidence: Ongoing trials across multiple cancer types continue to generate positive results
Technological Advancement: Next-generation approaches promise improved efficacy and manufacturing
Growing Infrastructure: Increasing number of centers developing TIL therapy expertise
Pipeline Momentum: Multiple TIL products advancing through clinical development
As research continues to optimize TIL therapy through patient selection, manufacturing enhancements, genetic modifications, and combination strategies this approach is poised to transform the treatment landscape for numerous solid tumors. The journey from laboratory bench to FDA approval took over 30 years, but the impact on patients' lives makes every year of research worthwhile.
For oncology professionals, researchers, and investors, TIL therapy represents a unique convergence of scientific innovation, clinical need, and commercial opportunity. The field stands at an inflection point, with the potential to help tens of thousands of cancer patients who currently have limited treatment options.
The TIL revolution is not coming it has arrived. The question now is not whether TIL therapy will transform solid tumor treatment, but how quickly and how broadly this transformation will unfold.
Frequently Asked Questions (FAQ)
Q1: How is TIL therapy different from checkpoint inhibitors like pembrolizumab or nivolumab?
A: Checkpoint inhibitors are antibodies that block inhibitory signals, allowing existing immune cells to attack tumors. TIL therapy provides billions of tumor-reactive immune cells that have been expanded outside the body. Many TIL patients have previously received checkpoint inhibitors, and research shows TILs can be effective even after these therapies stop working.
Q2: What is the success rate of TIL therapy?
A: In the FDA-approved melanoma indication, approximately 31.5% of patients experienced objective tumor responses, with complete responses in some patients. About 40% of responding patients maintained disease control for at least one year. Success rates vary by cancer type and patient characteristics.
Q3: How long does the TIL therapy manufacturing process take?
A: Manufacturing typically requires 3-4 weeks from tumor harvest to product availability. During this time, patients continue standard supportive care while their TILs expand in the laboratory.
Q4: Is TIL therapy covered by insurance?
A: As an FDA-approved therapy, lifileucel (Amtagvi) is generally covered by Medicare and most private insurance plans for the approved indication of advanced melanoma after prior therapy. Coverage specifics vary by plan and indication.
Q5: Can TIL therapy be repeated if the cancer returns?
A: Repeat TIL infusions are possible and being studied in clinical trials. Some patients may benefit from multiple TIL treatments, though this approach requires additional tumor harvests and manufacturing cycles.
Q6: What side effects should patients expect from TIL therapy?
A: Common side effects include those from lymphodepleting chemotherapy (low blood counts, fatigue, nausea) and IL-2 administration (fever, fluid retention, low blood pressure). Most side effects are temporary and manageable with supportive care during hospitalization.
Q7: How does TIL therapy work in cervical cancer compared to melanoma?
A: In cervical cancer, TILs often target HPV viral proteins (E6 and E7) expressed by cancer cells. Clinical trials show objective response rates around 44% in recurrent cervical cancer, with some patients achieving complete remissions lasting several years.
Q8: Are there age restrictions for TIL therapy?
A: Clinical trials have primarily enrolled adults (18+ years). Patient selection depends more on performance status, organ function, and ability to tolerate intensive therapy than on chronological age.
Q9: What happens if not enough TILs can be grown from my tumor?
A: Some tumor samples do not yield sufficient TILs for therapy. In such cases, additional tumor harvests may be attempted, or alternative treatment approaches considered. Manufacturing success rates vary by tumor type and individual factors.
Q10: How is TIL therapy monitored for effectiveness?
A: Patients undergo regular imaging studies (CT or PET scans) typically starting 4-6 weeks after TIL infusion and continuing at defined intervals. Response is assessed using standard oncology criteria (RECIST 1.1). Blood tests may also monitor immune cell populations and tumor markers.
References
National Cancer Institute. (2024). Lifileucel First Cellular Therapy Approved for Cancer. Cancer Currents Blog.
U.S. Food and Drug Administration. (2024). FDA approves first cellular therapy to treat patients with unresectable or metastatic melanoma.
National Cancer Institute SEER Program. (2024). Cancer Stat Facts: Melanoma of the Skin.
Chesney, J., Lewis, K.D., Kluger, H., et al. (2022). Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies. Journal of Immunotherapy for Cancer, 10(12), e005755.
Sarnaik, A.A., et al. (2021). Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. Journal of Clinical Oncology, 39(24), 2656-2666.
National Cancer Institute. (2024). Personalized immunotherapy shrinks solid tumors.
Stevanović, S., et al. (2015). Complete Regression of Metastatic Cervical Cancer After Treatment With Human Papillomavirus–Targeted Tumor-Infiltrating T Cells. Journal of Clinical Oncology, 33(14), 1543-1550.
National Institutes of Health. Recent advances in immunotherapy for cervical cancer.
ClinicalTrials.gov. Treatment Clinical Trials for Cervical Cancer.
National Institutes of Health. (2024). Tumor infiltration therapy: from FDA approval to next-generation approaches. Clinical and Experimental Medicine.
National Institutes of Health. Research progress on tumor-infiltrating lymphocyte therapy for cervical cancer.
National Institutes of Health. T cell immunotherapy for cervical cancer: challenges and opportunities.
National Institutes of Health. Adoptive tumor infiltrating lymphocytes cell therapy for cervical cancer.
ClinicalTrials.gov. Clinical Trials Using Tumor Infiltrating Lymphocyte Therapy.
National Institutes of Health. Advances and prospects in tumor infiltrating lymphocyte therapy.
National Cancer Institute SEER Program. (2024). Trends in Melanoma Incidence, Prevalence, Stage at Diagnosis, and Survival.
Centers for Disease Control and Prevention. (2024). Patterns and trends in melanoma mortality in the United States, 1999–2020.

